The McLafferty Rearrangement
In this tutorial I want to talk about the McLafferty rearrangement, a specific type of fragmentation for carbonyl compounds that we see in mass spectrometry.
McLafferty Rearrangement Mechanism
So, how does this rearrangement work?
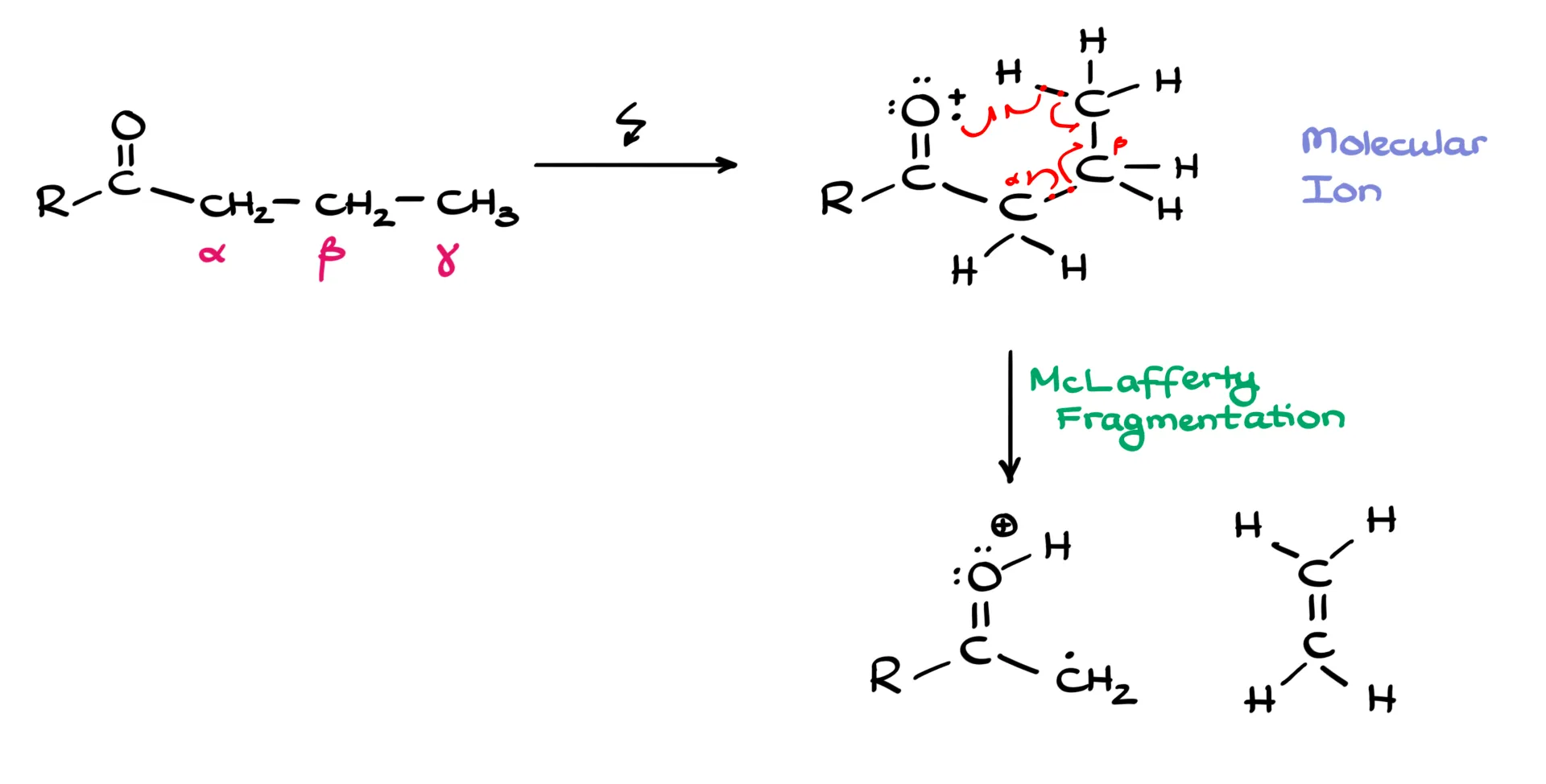
First, we need a carbonyl. Then we label the carbons next to it as the alpha, beta, and gamma carbons, written as α, β, and γ. We need at least a three-carbon chain, and the γ carbon must have at least one hydrogen. When we ionize the molecule, we first form the molecular ion, also called the ion radical, more precisely a cation radical. This species is unstable and decomposes in a very specific way, which is why I like to sketch it in a cyclic arrangement to show what is about to happen.
Mechanistically, we cleave the bond between the α and β carbons. There are different ways to depict the electron flow, but we usually show it with half-headed, fishhook arrows to emphasize the radical nature of this process. One electron from the carbonyl oxygen picks up the γ-hydrogen, that hydrogen shifts to the oxygen, an electron from the C–H bond moves to form a new π bond between the β and γ carbons, and the α–β bond splits with one electron staying on each side. As a result, we form two fragments. On the carbonyl side we have a cation radical in which the hydrogen that used to be on the γ carbon is now on the oxygen, and the adjacent carbon bears a radical that is stabilized by resonance with the carbonyl. The other fragment is an alkene. The alkene is neutral, so it flies away and we do not detect it. Mass spectrometry detects charged species only, so the fragment that contains the carbonyl and the charge is the one we will see.
tl;dr: whenever you see a carbonyl with a chain long enough to include α, β, and γ carbons, and at least one H on γ, a McLafferty rearrangement is possible. The molecule cleaves between α and β, and the oxygen-containing part is what shows up in the mass spec.
Example 1
Let’s check a couple of examples.
First, a simple ketone, 2-pentanone.
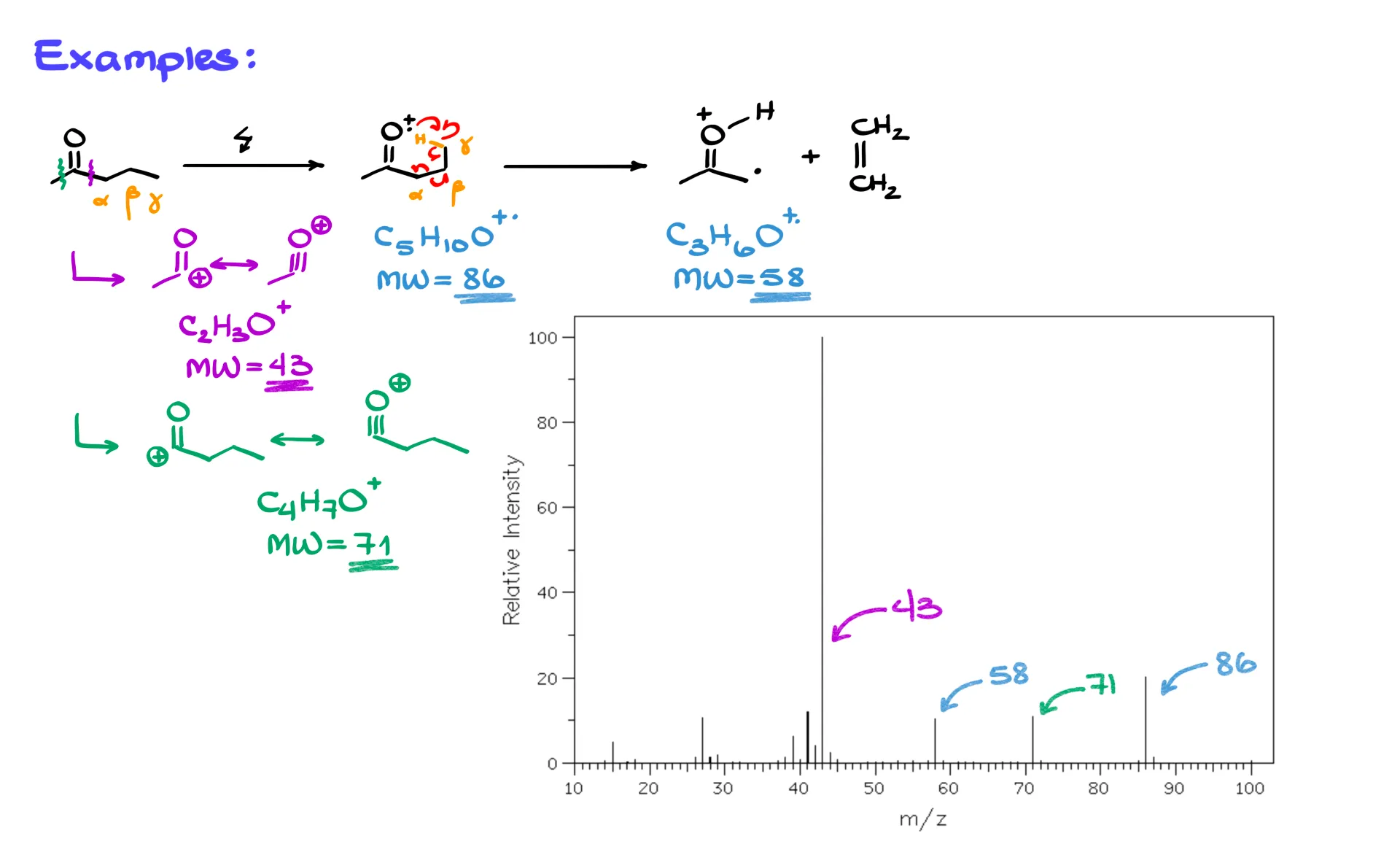
On the right of the carbonyl we have a three-carbon chain, so we can label α, β, and γ, and the γ carbon has hydrogens. In the mass spec, we first ionize the molecule to give the cation radical, and I like to draw it pre-organized for the rearrangement. From there, the fishhook arrows show the H transfer to oxygen, formation of the β–γ double bond, and cleavage of the α–β bond. The products are the cation radical on the carbonyl side and a neutral alkene.
Now the numbers. The molecular ion corresponds to C5H10O, which is m/z 86. The McLafferty fragment on the carbonyl side is C3H6O, which is m/z 58. These are not the only fragments you should expect. We also look for the most stable carbocations from normal cleavages. Cleavage between the carbonyl carbon and the α carbon gives a resonance-stabilized acylium-type cation, C2H3O+, at m/z 43. Cleavage on the other side of the carbonyl gives C4H7O+, at m/z 71. If your instructor is picky about notation, remember that the intact molecular ion and the McLafferty carbonyl fragment are cation radicals, while the 43 and 71 fragments here are carbocations.
Compare these predictions to the actual spectrum. You will see the molecular ion at 86, the McLafferty fragment at 58, and strong peaks at 43 and 71. If you relied only on “most stable carbocation” reasoning, you would account for 43 and 71, and maybe 86, but 58 would be hard to justify without invoking the McLafferty rearrangement.
Example 2
Now a slightly different example, an ester.
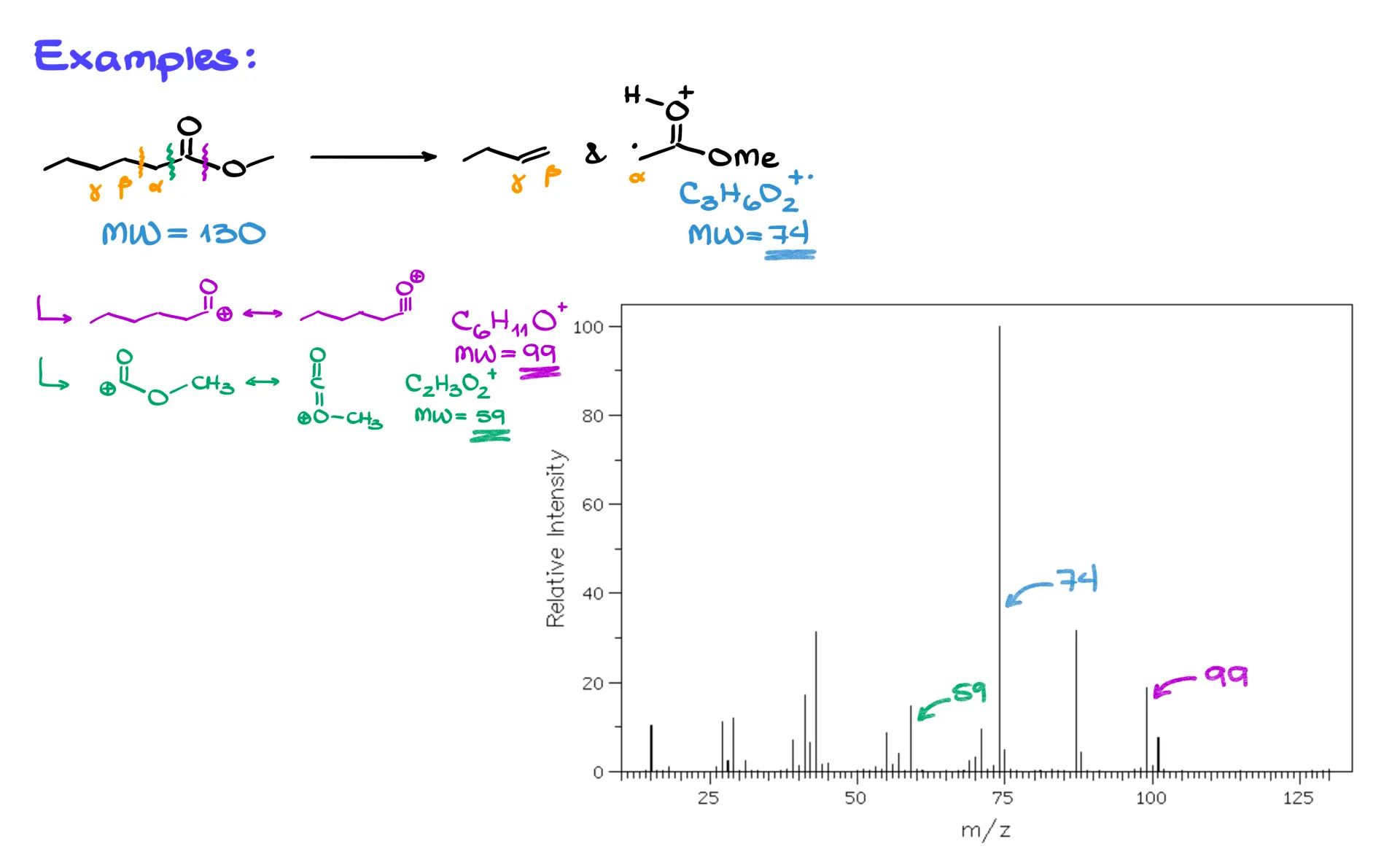
Again, we have a carbonyl with a longer chain, so label α, β, and γ. With at least one γ-hydrogen, the McLafferty rearrangement is available. The same electron flow applies, and we again detect the carbonyl-side cation radical and lose a neutral alkene. For this ester, the McLafferty carbonyl fragment is C3H6O2+. at m/z 74. The molecular ion of the parent ester is C7H14O2+. at m/z 130, although in practice that molecular ion may be weak or absent if the molecule fragments before detection.
Other common fragments appear as well. Loss of a methoxy group gives a resonance-stabilized cation C6H11O+ at m/z 99. A cleavage on the other side of the carbonyl can give C2H3O2+ at m/z 59. In the actual spectrum you will likely see 74, 99, and 59 clearly. The molecular ion at 130 may be missing if the ester is not stable enough under the ionization conditions, and since this molecule is larger than the ketone, you will also see many additional small peaks from further fragmentations.
So the McLafferty rearrangement lets us explain peaks that would otherwise be mysterious. In 2-pentanone the peak at 58, and in the ester the peak at 74, are classic McLafferty fragments. As molecules get larger, fragmentation trees become more complex, and multiple rearrangements can compete, but once you recognize the α, β, γ setup with a γ-hydrogen, you can predict this pathway and match the corresponding m/z values on the spectrum.
Reference: SDBS : https://sdbs.db.aist.go.jp/ , National Institute of Advanced Industrial Science and Technology, date of access: 10-11-25
