Topicity and Its Applications to NMR
In this tutorial I want to talk about group topicity. Whenever we talk about topicity in chemistry, we’re referring to the stereochemical relationship between atoms or groups within a molecule. There are four types of relationships we can expect here. The atoms or groups can be homotopic, enantiotopic, diastereotopic, or heterotopic.
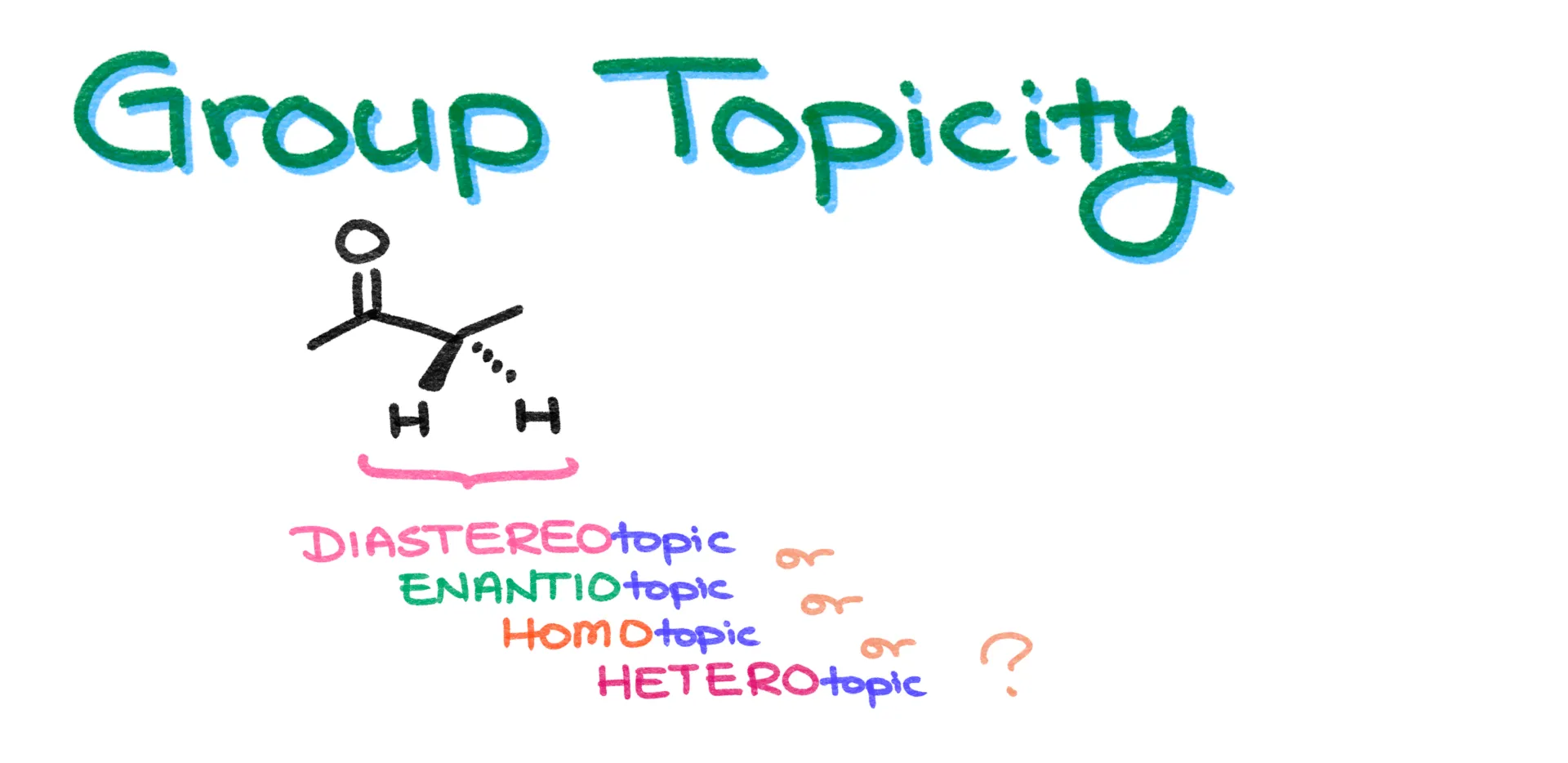
Now, trying to memorize the definitions of each without examples is pretty confusing. So instead, I’ll walk you through each type using actual examples.
Enantiotopic, Diastereotopic, Homotopic, and Heterotopic Groups
Let’s start with this molecule. I’ve highlighted three hydrogens because we’re going to focus on those. These three hydrogens are chemically identical. That means they are homotopic. We can prove that by replacing each hydrogen, one at a time, with an imaginary atom—let’s say X—and comparing the resulting molecules. If I replace the top hydrogen in one version and the middle hydrogen in another, the resulting molecules are exactly the same. Even if that X was something like a bromine or a wedge group, the molecules would still be identical. So, those hydrogens are homotopic.
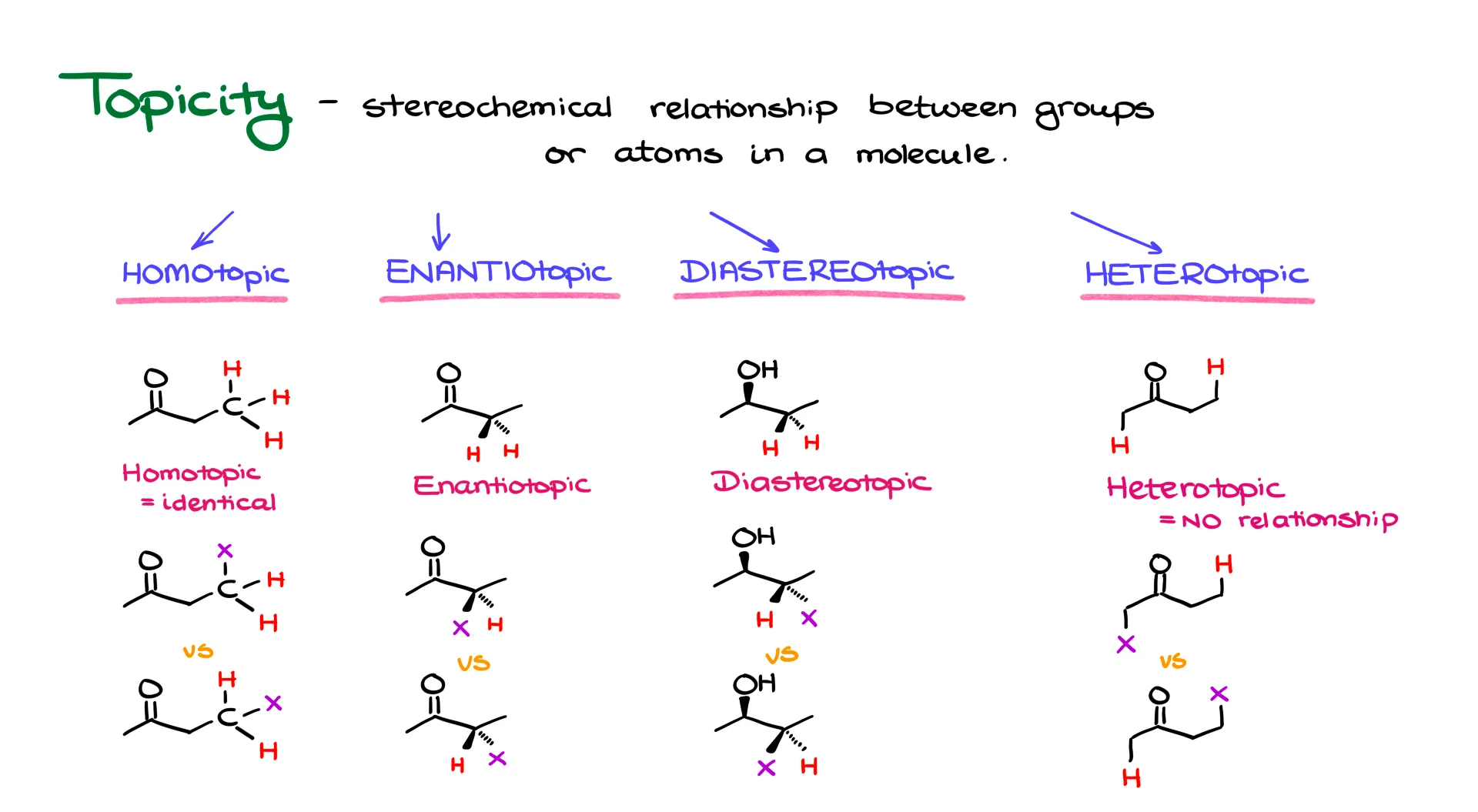
Now let’s look at the same starting molecule, but this time I’ll focus on two hydrogens in the middle of the molecule. Using the same X-replacement trick, I’ll replace one hydrogen in one structure and the other in the second. If we now compare the resulting molecules, we’ll see they are enantiomers. Because the molecules with the X are enantiomers, the original hydrogens are enantiotopic.
Next, I’ll return to the same starting material and focus on a different pair of hydrogens. Doing the same replacement, I end up with two molecules that are not even connected the same way—their atom connectivity is different. That makes them constitutional isomers, or structural isomers. There’s no stereochemical relationship here. So we call these hydrogens heterotopic. Heterotopic always means there’s no meaningful stereochemical relationship between the atoms or groups.
Now, let’s talk about diastereotopic groups. These are a bit more interesting, so I’ll use a different molecule as an example—a chiral alcohol. I’ll focus on two hydrogens in the middle. After replacing each hydrogen with X in separate molecules, the resulting structures are diastereomers. That means the original hydrogens are diastereotopic. As long as we can assign stereochemical relationships to the molecules after substitution, we can figure out the topicity.
More Examples
Let’s go through a few more examples to make this even clearer.
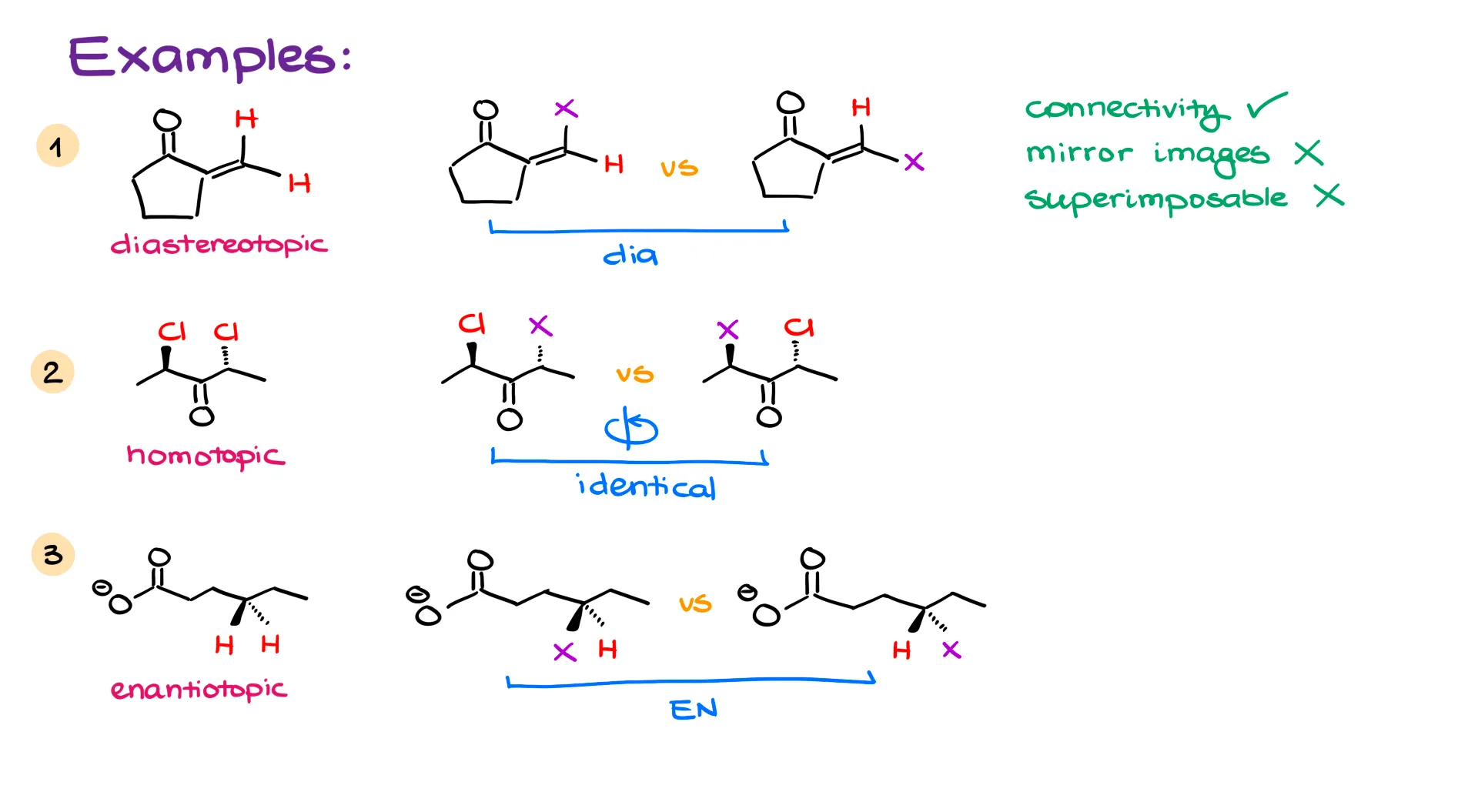
In my first one, I’m looking at two highlighted hydrogens. Step one is to redraw the molecule with one of those hydrogens replaced by X. Then I check for connectivity. If the connectivity is the same, they’re not heterotopic. The molecules aren’t identical either, so they’re not homotopic. That leaves us with either enantiotopic or diastereotopic. Are they mirror images? No. Are they superimposable? Also no. That makes them diastereomers, and the original hydrogens diastereotopic.
Keep in mind: we don’t need to have chiral centers to have enantiomers or diastereomers. The definitions only depend on whether the molecules are mirror images and whether they’re superimposable.
Next, let’s check the topicity of chlorine atoms in a molecule. I’ll replace one chlorine with an X and analyze the resulting pair. If I can rotate one molecule into the other, they’re identical. And if the molecules are identical, that means the original chlorines are homotopic.
Now I’m back to comparing hydrogens. Replace each hydrogen with X, and if the resulting molecules are enantiomers, then the original hydrogens are enantiotopic. So again, follow the same process: replace one group, check what you get, and figure out the relationship.
Here’s a summary of the algorithm: replace the group of interest with X. If the resulting molecules are unrelated and have different connectivity, the groups are heterotopic. If they are identical, the groups are homotopic. If they’re diastereomers, the groups are diastereotopic. If they’re enantiomers, then they’re enantiotopic.
Application of Topicity to NMR Spectroscopy
Now, this might seem like a weird detail of stereochemistry—why does this even matter? Here’s why: group topicity is extremely useful in spectroscopy, especially in ¹H NMR.
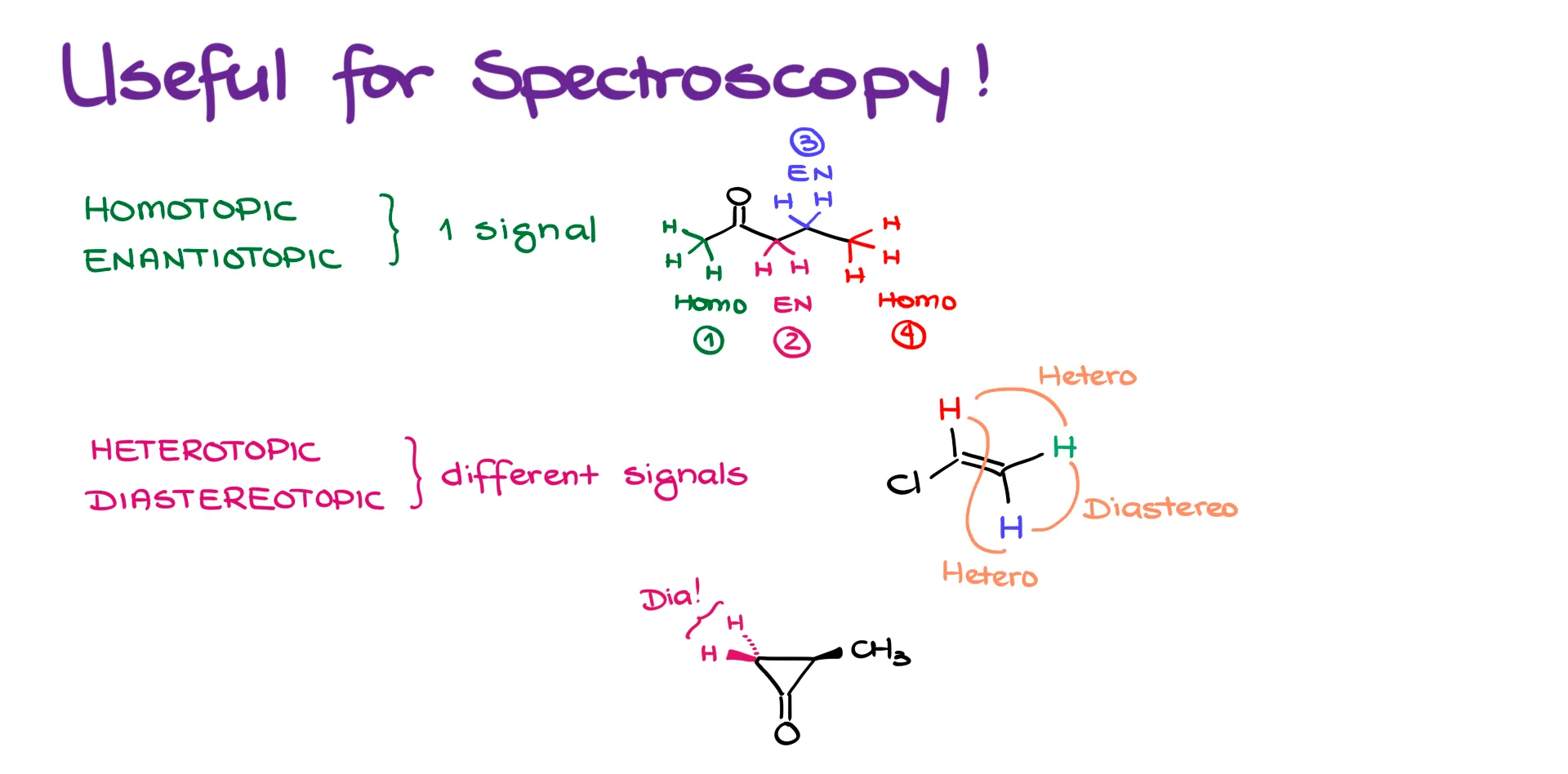
If your atoms or groups are homotopic or enantiotopic, they’ll show up as one signal in the ¹H NMR. For instance, in this molecule, the three hydrogens on the first methyl group are homotopic, so they’ll produce one signal. The two hydrogens on the adjacent carbon are enantiotopic and give the second signal. The next pair of hydrogens are also enantiotopic and give the third. Finally, the last methyl group’s hydrogens are homotopic again and give the fourth signal. In NMR, all hydrogens within each group behave identically.
However, if the groups are heterotopic or diastereotopic, they are not the same and will appear as different signals in the NMR. Take vinyl chloride for example. The two hydrogens on the double bond are heterotopic, and so are the next two hydrogens. The green and blue hydrogens are diastereotopic. This means you’ll get three unique signals—each hydrogen is distinct in this spectrum.
Here’s another example. If I look at two highlighted hydrogens on the same carbon, and they’re diastereotopic, they will show up as different signals in the NMR. That’s the key. Even if hydrogens are on the same atom, like the same carbon, they can give different signals if they’re diastereotopic.
So anytime you’re unsure whether hydrogens or even carbons are going to appear the same or not in an NMR spectrum, go through the topicity process. If they are homotopic or enantiotopic, they will be identical in NMR. If they are heterotopic or diastereotopic, they will give different signals.
Also, since heterotopic and diastereotopic atoms are distinct, they can even split each other’s signals in NMR. I know it sounds strange, but if you have two diastereotopic hydrogens sitting on the same carbon, they absolutely can and will split each other’s signals.
And that’s group topicity—essential for understanding how to interpret your NMR spectra properly!
