Enolization & Keto-Enol Tautomerism
Enolization or a keto-enol tautomerism is a process of converting a ketone or an aldehyde to a corresponding enol (in acidic conditions) or an enolate (in basic conditions). This process can occur in esters as well. I’m going to focus on aldehydes and ketones in this post, however, so I don’t overload it with the details and nuances.
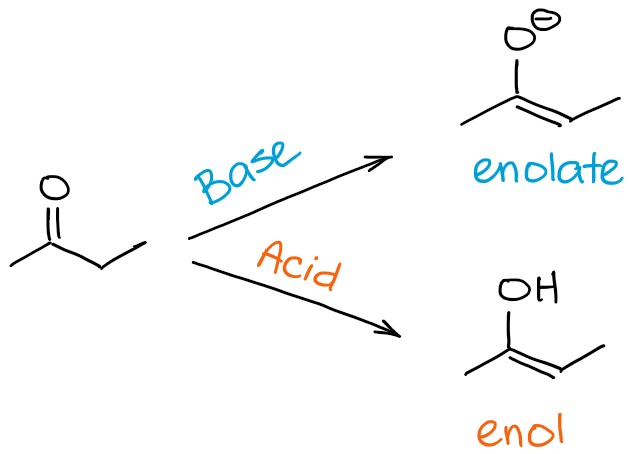
So, what’s the difference between an enolization and a keto-enol tautomerization processes? A short answer is none really 😊
When we refer to the enolization, we specifically refer to the process that takes you from a ketone or an aldehyde to an enol or an enolate. When we refer to a keto-enol tautomerism, we mean the equilibrium in general. Thus, by saying that my molecule undergoes the keto-enol tautomerization, I may imply the direction of the process, but I generally don’t point it out per se. The direction of the keto-enol tautomerization, however, can often be deduced from the context of the reaction.
In a nutshell, if you say that molecule “A” undergoes an enolization, it means that “A” becomes an enol or an enolate. If you say that a molecule “B” undergoes keto-enol tautomerization, it may mean enolization if B is a carbonyl, or it may mean a reverse process if B is already an enol or an enolate.
Base-Catalyzed Enolization
While enolization can occur in either basic or acidic conditions, base-catalyzed enolization is a more common way to do this reaction. Thus, I think it’s a good idea to go over the enolization in basic conditions first.
In basic conditions, a ketone or an aldehyde acts as a Brønsted acid donating an proton from an α-carbon to a base in the solution. This process gives a resonancely stabilized enolate anion:

Since in this equilibrium our carbonyl acts as an acid, it needs to be sufficiently acidic to give away the proton. Alternatively, the base needs to be sufficiently strong to drive this equilibrium towards the product.
How Acidic Are Hydrogens on the α-Carbons?
The enolate anion is a resonancely stabilized species, so the extent of the resonance stabilization plays a very significant role in the acidity of carbonyl compounds. We know, that the more resonance contributors we can draw for the conjugate base, the more stable it is. And the more stable the conjugate base, the more acidic the original acid is. Thus, hydrogens in between two C=O bonds will be significantly more acidic than the ones next to only one C=O.
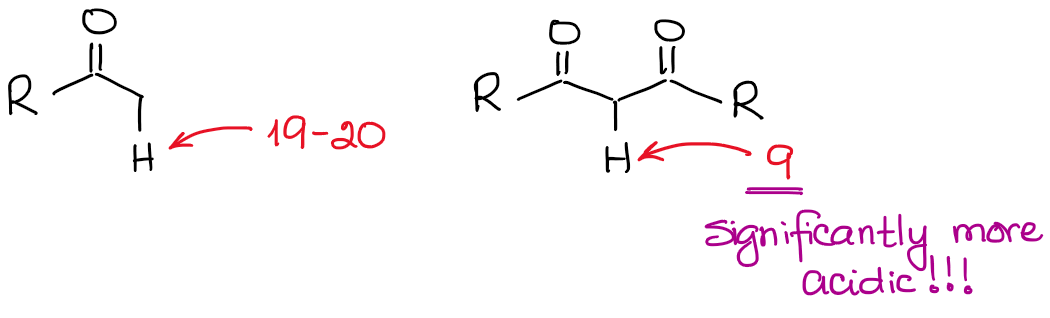
If we compare the conjugate bases in both cases, we’ll see that the dicarbonyl has a better resonance stabilization of the negative charge through a more extended conjugated system.
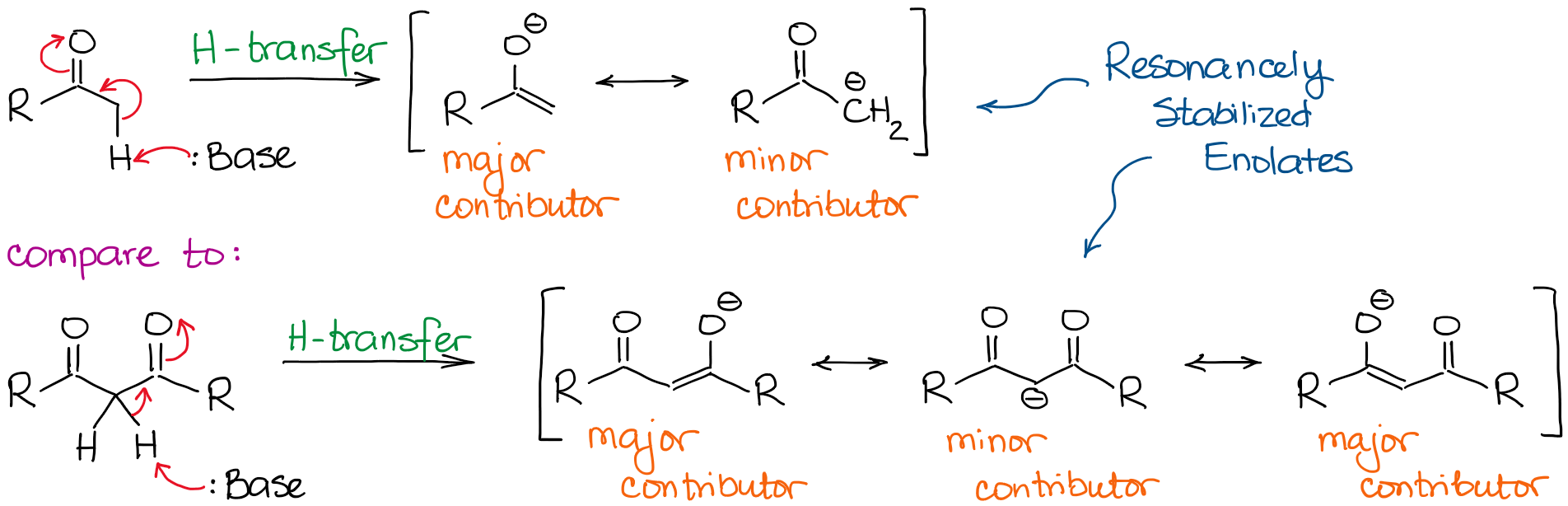
An additional major resonance contributor in the dicarbonyl anion makes the molecule about 10000000000 times more acidic! So, the nature of a ketone or an aldehyde is fairly clear here: the more C=O bonds next to a hydrogen, the more acidic it becomes.
Effect of a Base on the Enolization Equilibrium
Bases have vastly different strengths. So, the choice of a base is important if you want to drive your equilibrium to the completion. Let’s look at a few bases we typically see in organic chemistry reactions:
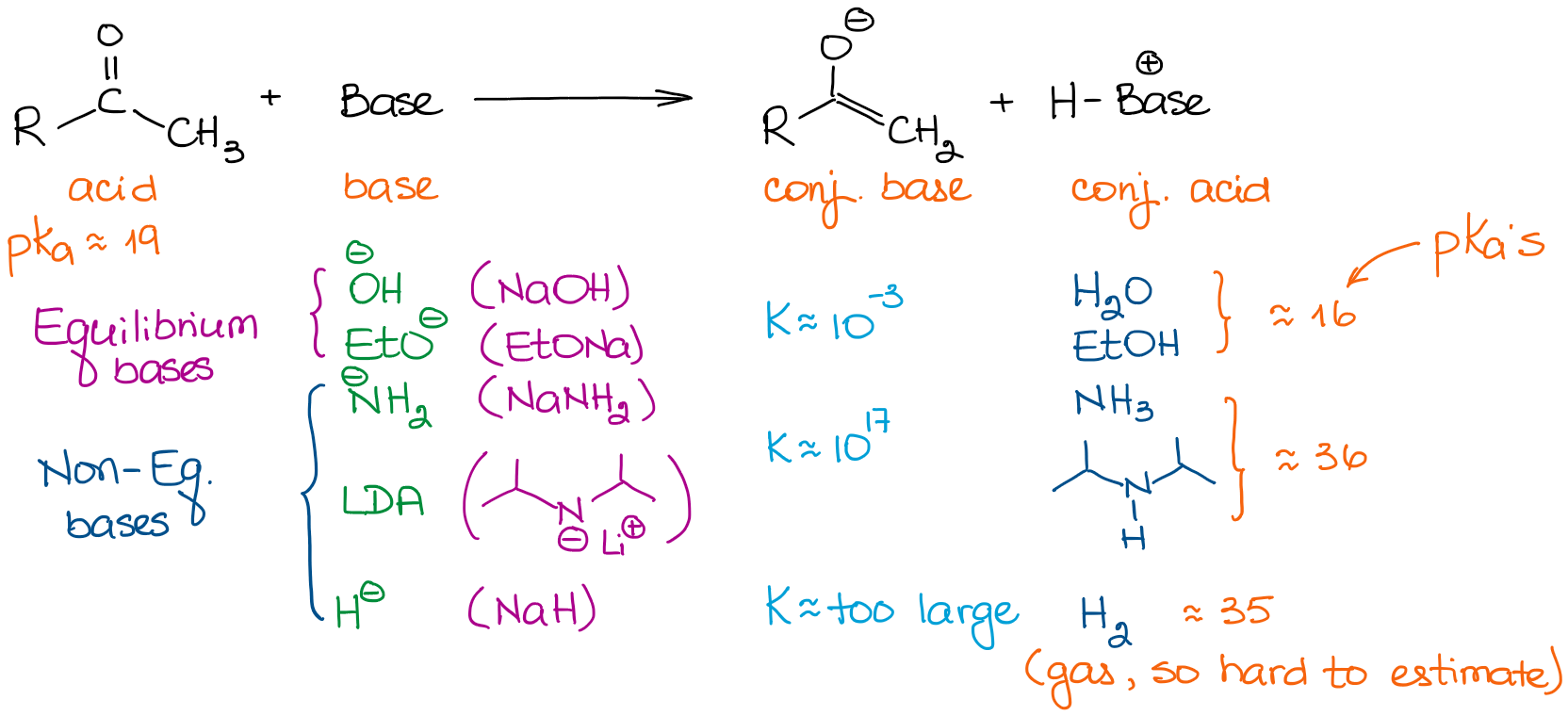
When we are dealing with the simple aldehydes and ketones, we can classify all bases into two categories: equilibrium and non-equilibrium bases. The equilibrium bases are relatively weak. This means that they can only drive the equilibrium to a very modest extent. Thus the actual concentration of an enolate in the solution will be very small. The reaction will also be in a constant equilibrium between the starting material and an enolate. These bases favor the formation of more thermodynamically stable enolates. These enolates are called thermodynamic enolates.
Non-equilibrium bases, however, are very strong. This leads to a proton transfer (acid-base) reaction with very large equilibrium constants. When we have an equilibrium constant over 103, the equilibrium is pretty much completely shifted towards the products. So, an enolization with a base like LDA gives virtually 100% enolization. Thus, the concentration of the initial aldehyde or a ketone is basically zero.
Carbonyls with One C=O Bond vs β-Dicarbonyls
As I’ve mentioned above, β-dicarbonyls are significantly more acidic than regular aldehydes and ketones. Let’s look at an equilibrium constant in the following two cases:
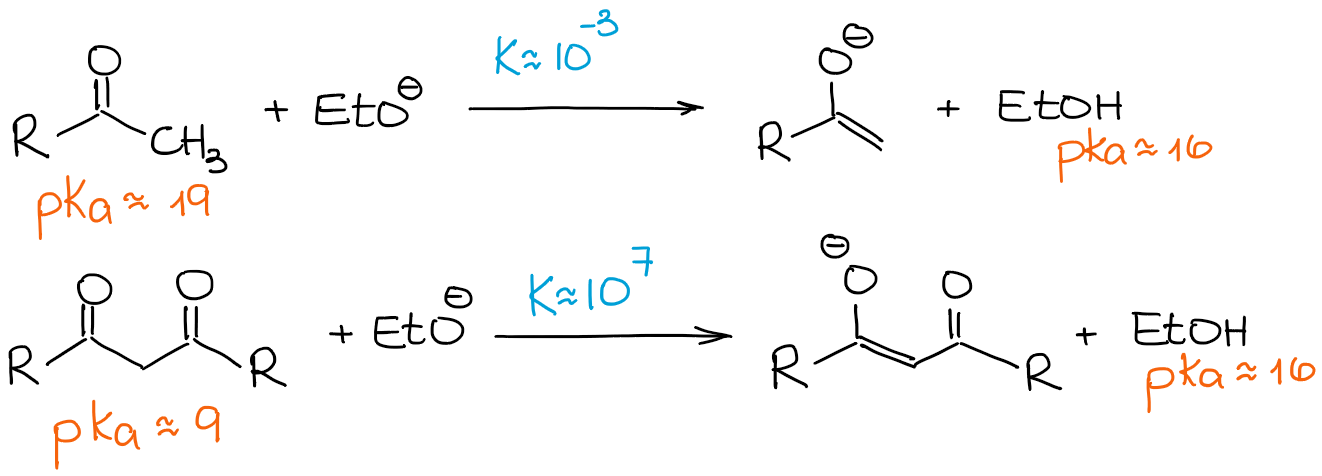
Since a β-dicarbonyl is so much more acidic than a regular aldehyde or a ketone, even a weak base drives the equilibrium towards the products giving 100% enolization. So, we’re going to look at any enolization reaction of the β-dicarbonyls as a 100% enolate and 0% starting material process regardless of a base.
Why the Choice of a Base is Important?
Some reactions can be very sensitive to what’s in the solution and what are the concentrations of the reactants. For instance, aldehydes are electrophilic while the enolates are nucleophilic. So, if I’m using a weak equilibrium base which leaves a lot of the starting material in the solution, I may start seeing an undesirable reaction between an enolate and an initial aldehyde. Generally, we’re only going to see the weak equilibrium bases used when we are not going to expect much of a competition between the possible products. If multiple reactions are possible and no single major product dominates the equilibrium we’ll always use strong non-equilibrium bases.
Bulky vs Small Bases
The size of a base can be a determining factor in the enolization process when multiple enolizable positions in a molecule are competing. Let’s look at the enolization of 2-methylbutanone by two strong bases:
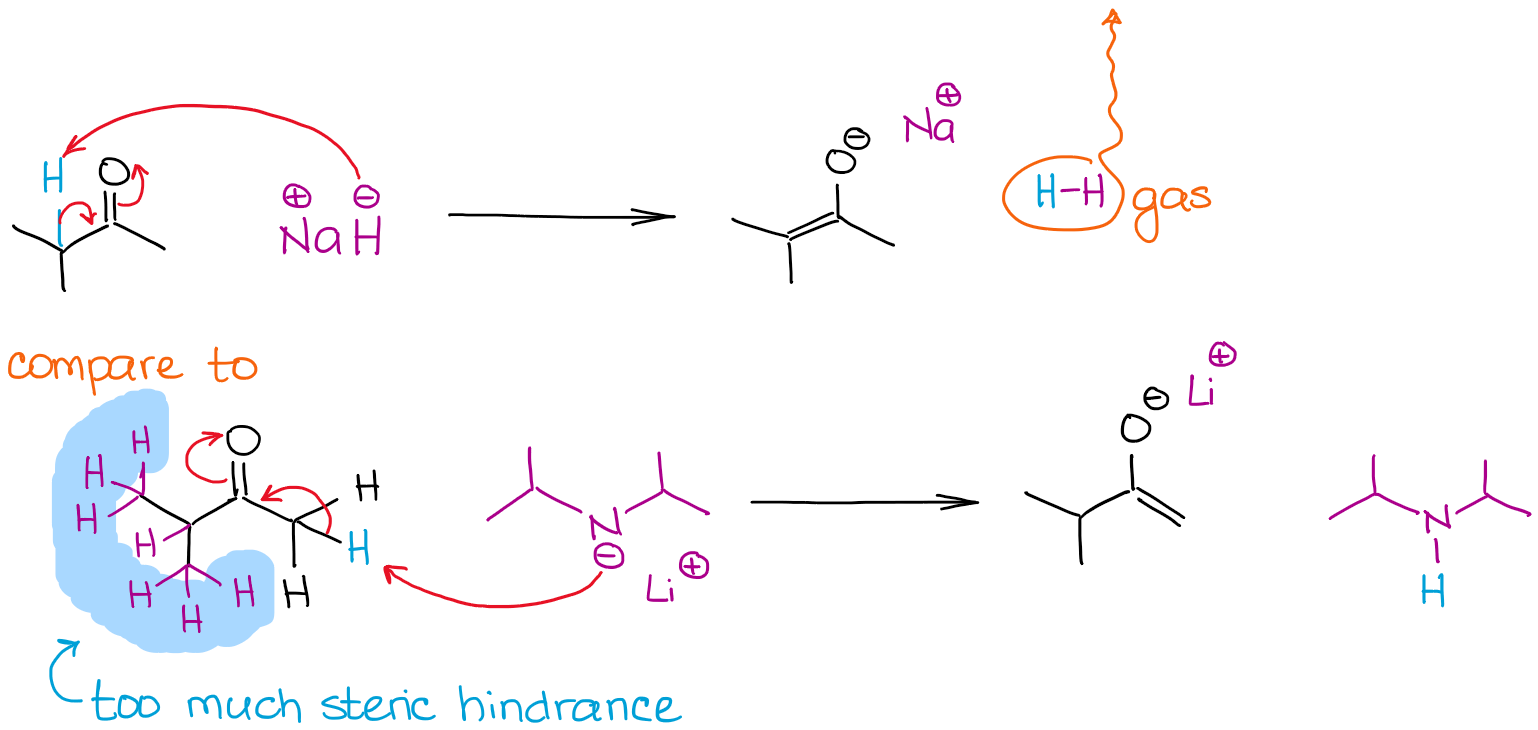
Both reactions give 100% enolization due to the use of very strong non-equilibrium bases. However, the reaction with sodium hydride (NaH) gives a more thermodynamically stable enolate. Unlike LDA, hydride anion is very small so it can easily reach a more sterically hindered proton and give a thermodynamic enolate. LDA is very bulky and thus is very sensitive towards any steric hindrances. This means, that it will go after less sterically hindered and more accessible enolizable hydrogens.
We call the less thermodynamically stable enolate like in the reaction with LDA a kinetic enolate. This is also where the reagents addition order becomes relevant. To get a kinetic enolate, you should always add a carbonyl to the base, and not the other way around! Some instructors are picky about it, so keep that in mind.
Thermodynamic vs Kinetic Enolates
By now I’ve mentioned thermodynamic and kinetic enolates a few times, so I think it might be a good idea to go over those in a bit more details.
What exactly counts for the thermodynamic stability of an enolate?
Major enolate resonance contributors have a negative charge on the oxygen atom and a double C=C bond.
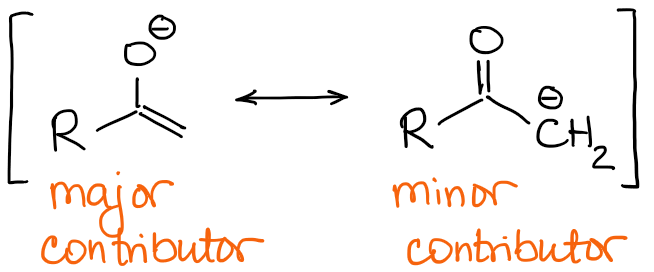
Thus, the nature of the double bond will be the main difference between the enolates. If we look back at the example with 2-methylbutanone from above, we can see the two possible enolate have a qualitatively different double bonds:
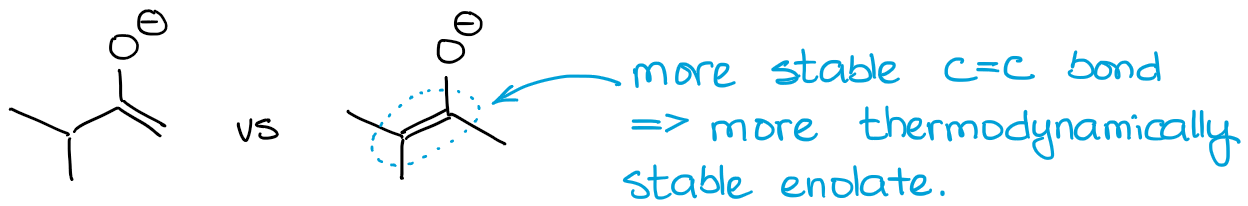
Always look for the number of substituents on your double bond. The more substituents you have on a double bond, the more stable it is. Thus, the thermodynamic enolate will always have the busiest double bond.
A kinetic enolate is the one that forms quicker. Think about the reaction with LDA. LDA is a very strong non-equilibrium base. As soon as it snatches off the proton, it’s not going to give it back. It’s also a very bulky base and it can’t grab a hydrogen from the middle of the molecule. So, it’s gonna go after what’s more sterically accessible one. Thus, a base like LDA snatches a proton fast, from a less sterically hindered site, and doesn’t give it back or allows for the equilibrium yielding a less stable enolate.
Why is it important to add a carbonyl to a base to make a kinetic enolate?
If you want a kinetic enolate, your base must always be in excess. Enolates are basic themselves! So, if you have a carbonyl in excess, then the enolate can quickly establish an equilibrium with it.
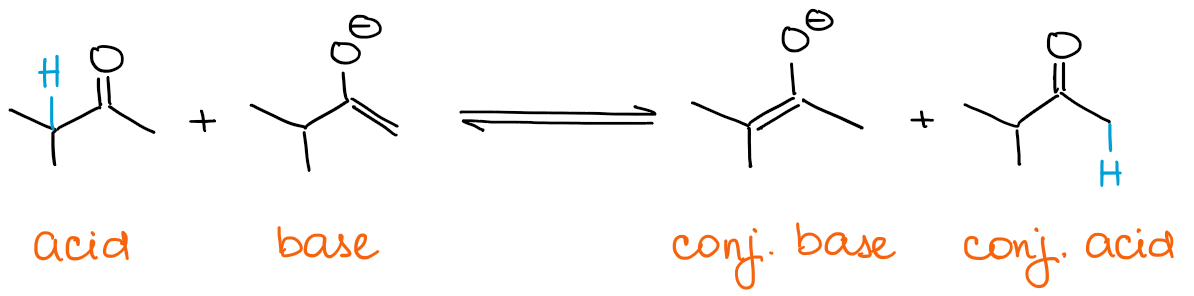
Any equilibrium will always favor more thermodynamically stable species. This means that you will always be getting a thermodynamic enolate if you have an excess of a carbonyl and not a base.
So, remember that:
Weak and small strong bases = thermodynamic enolate
Bulky strong bases = kinetic enolate
Using this ↑ simple rule of thumb will help you in determining what enolate you’re going to have or need to make in your reaction.
Acid-Catalyzed Enolization
At the very beginning of this post, I’ve mentioned that the enolization is also possible in acidic conditions. Since aldehydes and ketones are not particularly basic, you wanna use a very strong acid to catalyze this process. Typically, we use sulfuric or tosylic acids as catalysts.
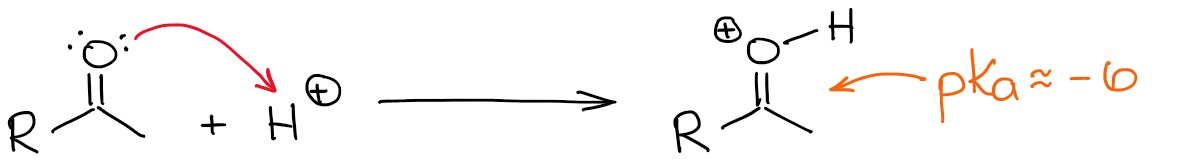
The most acidic hydrogen in a protonated carbonyl is, of course, the one on the oxygen. However, making a C=O bond more polarized by adding a proton to it, makes the α-position more acidic as well. So it’s now somewhat easier to deprotonate the α-position and make an enol.
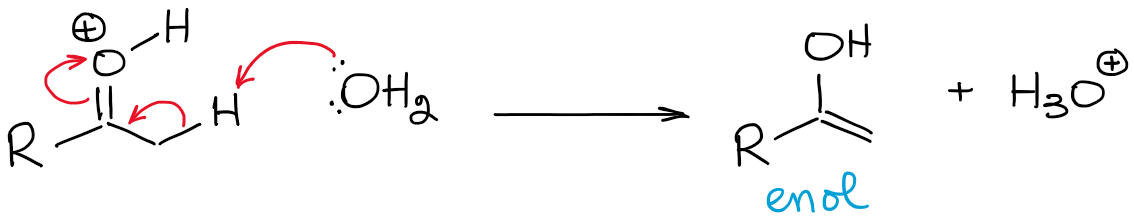
This equilibrium is hugely unfavorable and favors the carbonyl overall. Thus, you will never have a high concentration of an enol in acidic conditions unless there’s some kind of a stabilization effect making enol favorable (rare).
While all possible enol products can be produced, this reaction will always favor the formation of a thermodynamic enol.
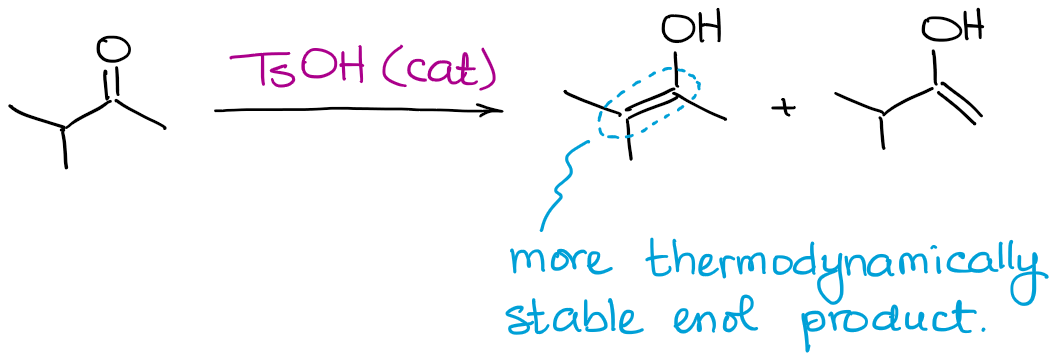
Just like in the case with enolates, a thermodynamic enol is the one that has more substituents on the double bond.
What factors influence acid-catalyzed enolization?
Anything that can make an enol more stable will help the equilibrium. Most common factors are the hydrogen bonding and extended conjugation.
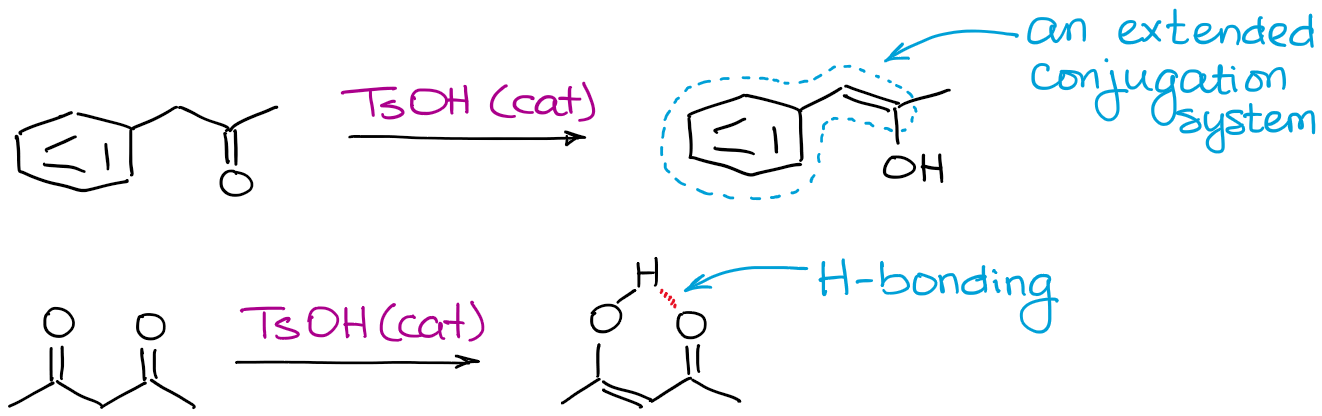
Why bother with acid-catalyzed enolization if there are so many issues with it?
Your compound might be sensitive to bases. Also, it might be easier to combine multiple steps when they occur in the same conditions. We often combine acid-catalyzed enolization with other steps in a reaction that also occur in acidic conditions.
You gotta keep in mind though, that electron-withdrawing groups in the vicinity of a carbonyl will make it even less basic than it already is. So, if you have, say, a halogen in the α-position, it will be really difficult to force the formation of an enol.
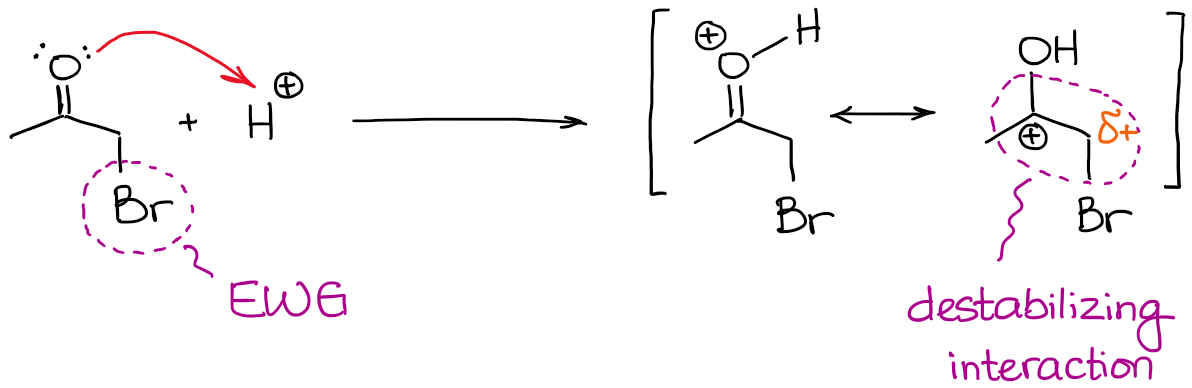
Always check for any EWG’s in the α-position before you assume any enol formation! Any EWG’s that do not offer resonance stabilization to the double bond will significantly disfavor the enolization process.
What to Expect on the Exam?
Professors rarely ask questions specifically targeting the formation of enols or enolates directly. Enolization questions are always going to be disguised as something else. Enolization questions are typically phrased as:
- Find the most acidic hydrogen in the following molecule… or
- Draw the product… (the reaction will have an enol or an enolate as an intermediate)
Of course, they might ask a direct question. However, in my experience, the keto-enol tautomerization and enolizations are usually the underlying concept that drives a certain question.

In which medium tautomerism will be faster ?????
Acidic or Basic ?
Neither. It depends on how the reaction is actually preformed and the nature of your reagents.
I think there is an error in “why the choice of a base is important?”
It’s “weak equilibrium base” and not “weak non-equilibrium base”
Good catch, thank you for pointing it out! I fixed it. It’s easy grow “blind” to some of those things when you’re looking at the text for a long periods of time when writing it and your brain just substitutes what needs to be there lol I appreciate you paying attention!
You are welcome! Thank you for the exaustive compendium on enolization, very useful and comprehensive.