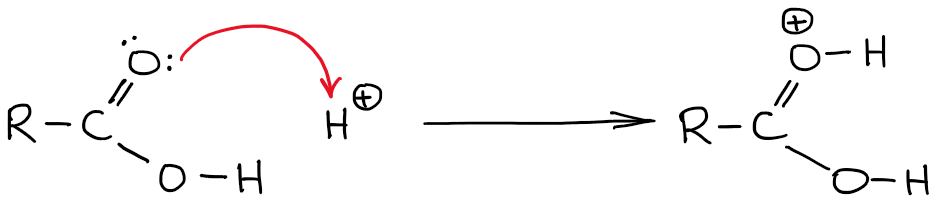
Protonating A Carboxylic Acid: Which Atom To Choose?
Carboxylic acids get protonated in a number of reaction mechanisms in organic chemistry. An example of such a reaction would be Fischer esterification. The question that I often discuss with my students in our tutoring sessions is which atom to protonate? After all, there are two oxygens and each of those is a potential acceptor of a proton an acid-base proton transfer.
If you’re in impatient type, I’ll give you a hint: it’s the C=O oxygen that gets the H. Now, let’s talk about the reasons behind why that’s the case.
The Carboxylic Acid Functional Group
First, we should consider the carboxylic acid functional group itself. It has two resonance contributors: one major and one minor:
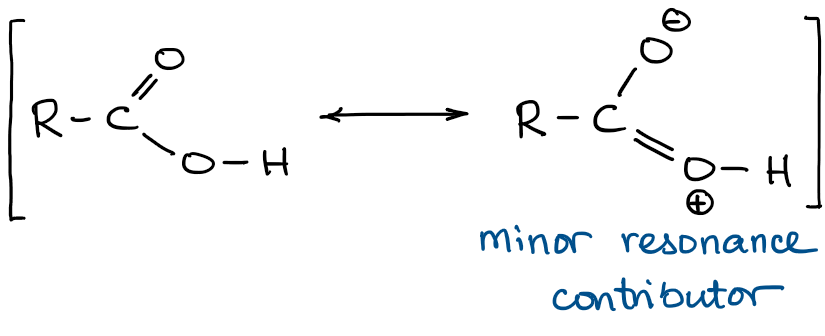
The right contributor is minor because it creates unnecessary charges in the molecule. However, it is a contributor, which means that it does have some impact on the overall resonance hybrid in the carboxylic acid. By superimposing both contributors we get the following hybrid:
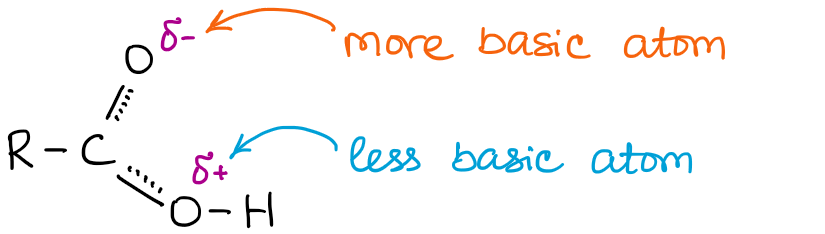
We can clearly see that the C=O oxygen is more basic simply due to the virtue of having a small partial negative charge, which means that it has some “excess” electron density and can easier share it with a proton to make a new bond.
Comparison of the Protonated Carboxylic Acids
Second. Now, let’s consider what we get when we actually do protonate a carboxylic acid.
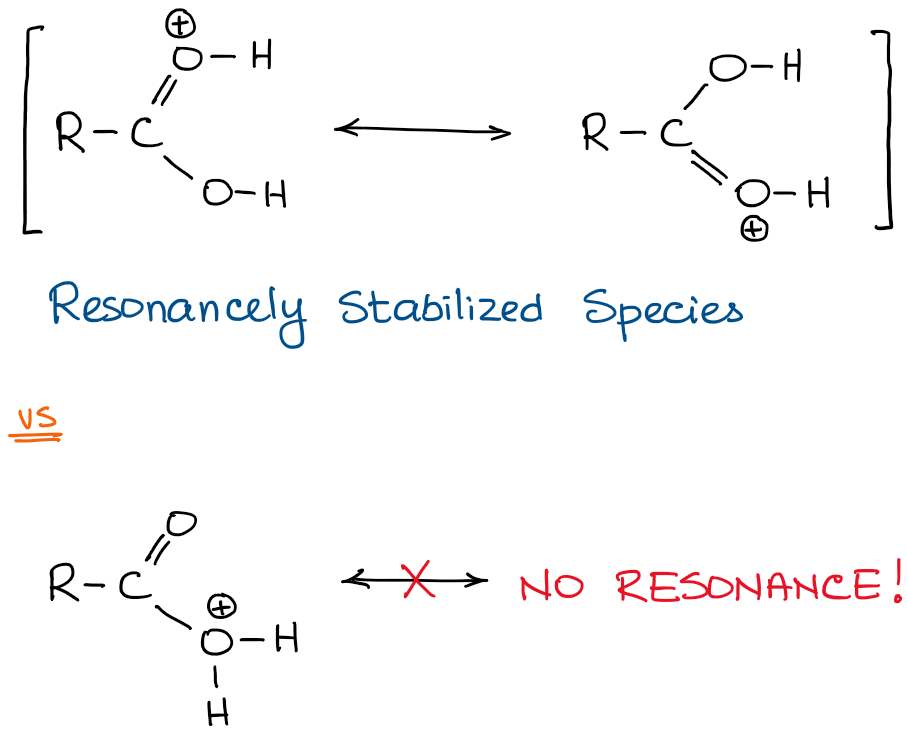
When the oxygen of the C=O bond is protonated, we get a resonancely stabilized species which offers a benefit of delocalization of electron density to make it more stable. The protonation of the -OH, however, does not offer any other “perks” in the form of resonance stabilization.
On top of that, there’s a destabilizing interaction between the protonated oxygen and partially positive carbon of the C=O:

You might protest by saying: “But hey, that makes a good leaving group!” And you would be absolutely correct: it does make a good leaving group. This is a flawed logic however, as the molecule does not “know” that it needs a leaving group for the next step of the reaction. The only thing that the molecule “knows” is how stable or unstable it currently is and how stable or unstable it becomes when you do something with it.
A molecule does not have long-term planning skills! It only “knows” how stable it is right now and how stable it will be when you do the action you’re considering! Never base your mechanism on the next step!
So, make sure you’re analyzing the present state of your system and NOT the futures steps. This is one of the most common misconceptions that I battle on a daily basis with my students: do not ever try to fit the mechanism to your needs! This is a very dangerous approach to mechanistic thinking and will lead you to an eventual trap.
Can the unfavorable protonation happen at all?
Absolutely! As the matter of fact, in a solution everything that can be protonated, will eventually get protonated. So you can think about the two possibilities as an equilibrium with the C=O protonated oxygen being a predominant species in this equilibrium:
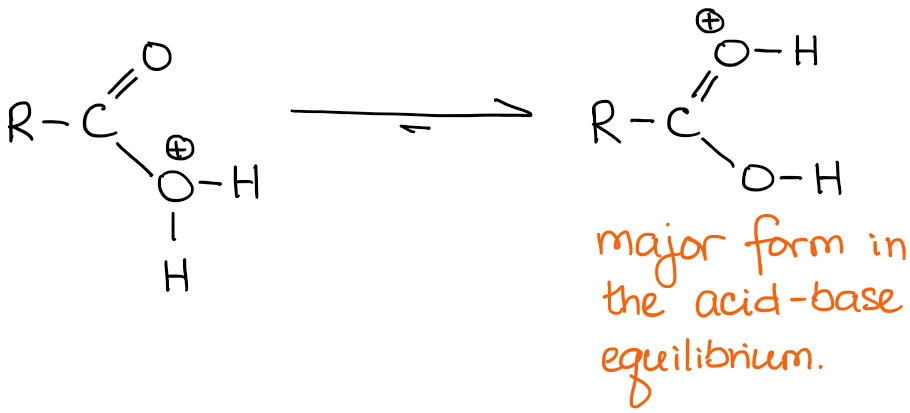
For the purposes of the mechanism writing, however, we will always be showing the C=O protonated version, so make sure you always write that on your exams.