Stereospecific vs Stereoselective Reactions
There are two terms that describe the stereochemical outcome of reactions. These terms are stereospecific and stereoselective. And due to the similarity in the terms, many students have a hard time remembering which one is which or even know the difference. So, let’s look at these two terms in a little more detail.
Stereoselective Reactions
These are reactions that can give two different stereoisomers but one of those stereoisomers is major while the other one is minor. Let me give you an example by looking at two different epoxidation reactions:
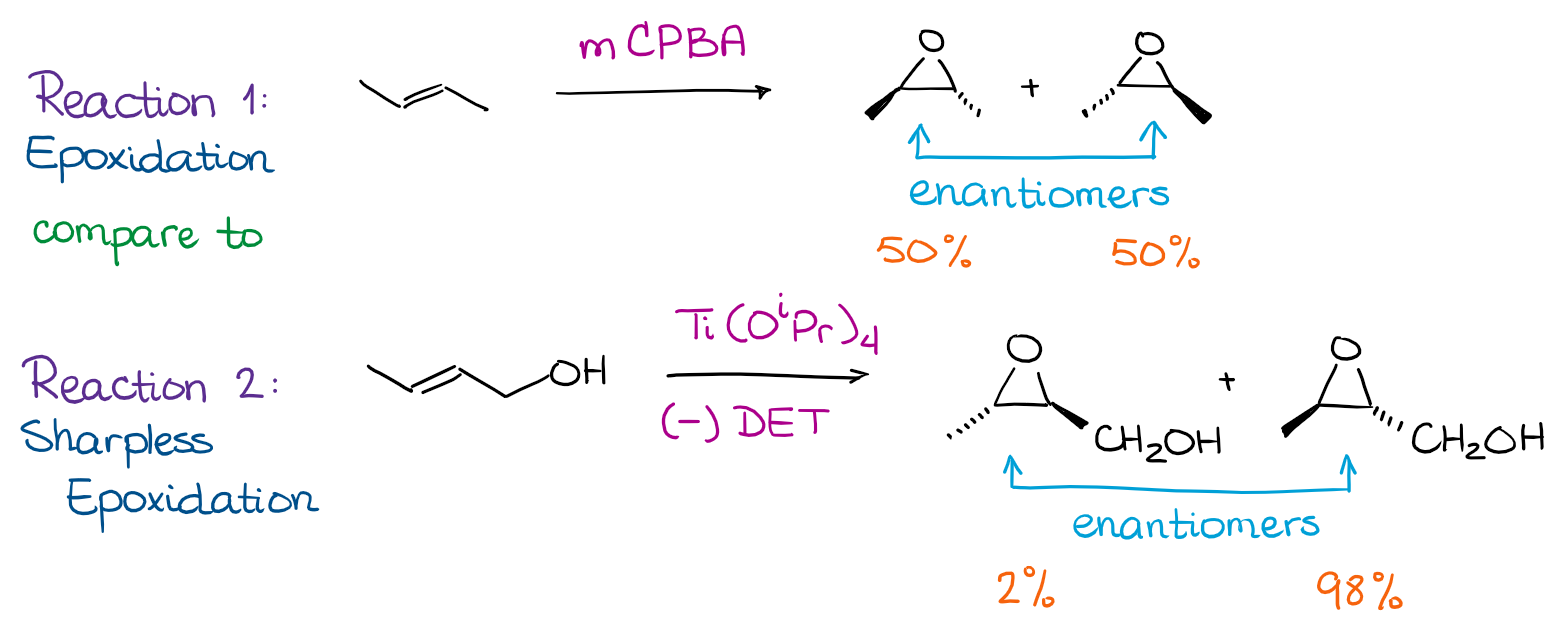
The first reaction gives you two stereoisomers. Those are enantiomers. The first reaction gives no preference to one stereoisomer or the other. So, it’s NOT stereoselective. The second one, however, is more selective towards one stereoisomer. Thus, we call it stereoselective. Since the stereochemical relationship between the products in the reaction is enantiomers, we can also call this reaction enantioselective. When the stereochemical relationship between the products is diastereomers, we call such a reaction diastereoselective.
Generally, within the scope of the sophomore organic chemistry course, we mention the stereoselectivity, but don’t focus on it. Stereoselective reactions, such as Sharpless epoxidation, are incredibly important in organic synthesis. The stereoselective methods and the mechanisms of those reactions, however, are usually quite complex. So, we simply don’t have time to go over all of those in an introductory course. If you’re interested in stereoselective methods in organic chemistry, you should take an advanced organic chemistry course.
Stereospecific Reaction
Many reactions we cover in the organic chemistry are stereospecific. When you hear the word “stereospecific” you should think about the “syn” and “anti” addition reactions. While there are other types of stereospecific reactions in organic chemistry, we traditionally only cover a few of those within the scope of the course. So, for instance, the halogenation of alkenes is an anti-addition reaction:
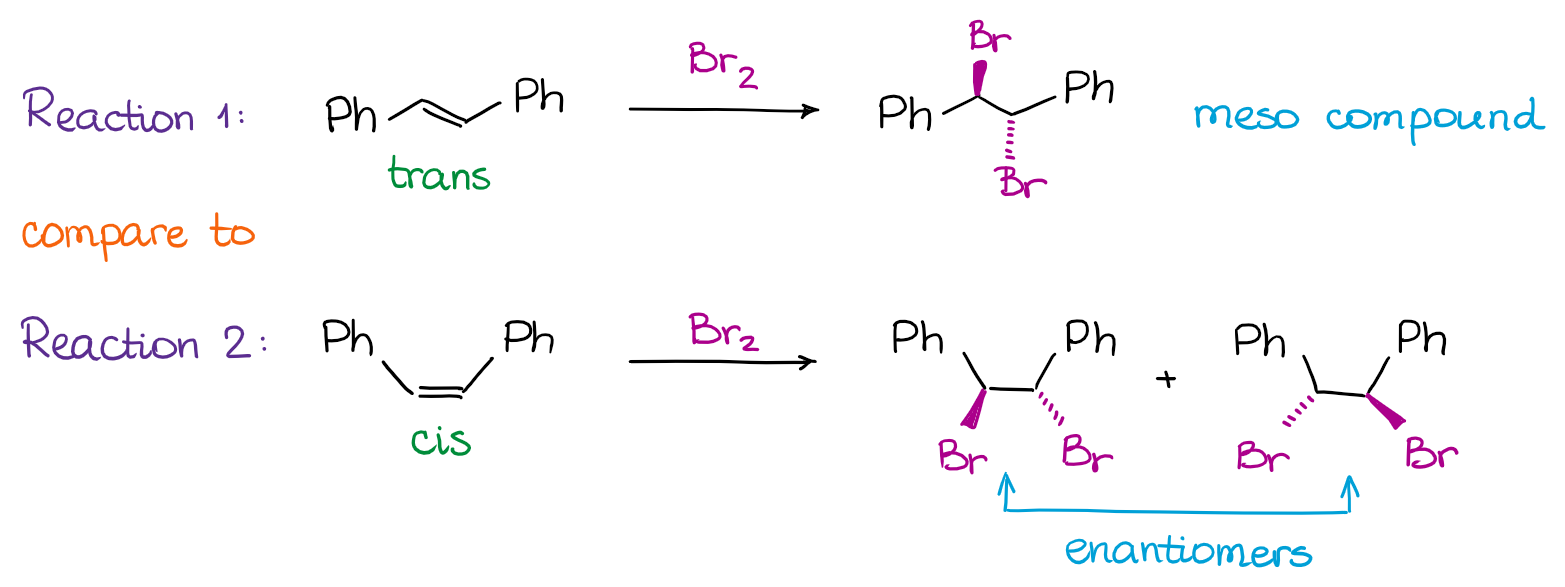
In the example above, we have a situation when a specific stereoisomer of the starting material (trans-stilbene) gives a specific stereoisomer for a product (a meso compound). Likewise, the cis-stilbene starting material, specifically, gives the pair of enantiomers. Notice, that due to the stereospecificity of the halogenation reaction mechanism (anti-addition), the trans-stilbene will never give you a pair of enantiomers while the cis-stilbene will never give you the meso compound. So, essentially, if the reaction is stereospecific and the starting materials are diastereomeric, the products will also be diastereomeric.
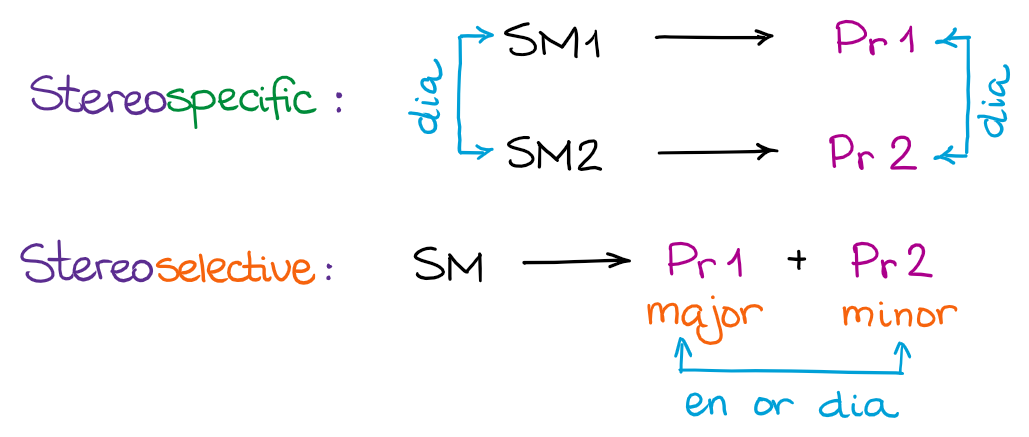
I suggest you make a quick flashcard of the scheme above and use the terms every time you look at the reaction to make sure you solidify the terminology and can use it comfortably whenever you need. Something very important to keep in mind: stereospecific is the description of the reaction mechanism, while stereoselective is the description of the reaction outcome! Thus, the reaction can be both stereospecific and stereoselective since the terms describe different aspects of the reaction.
