Mass Spectrometry Practice Problems
In this tutorial, I wanna go over a few more mass spectrometry practice problems. If you haven’t seen my previous tutorials on mass spectrometry and the McLafferty rearrangement, I’d recommend starting with those first and then coming back here to do a little more practice.
Example 1
Alright, let’s jump in.
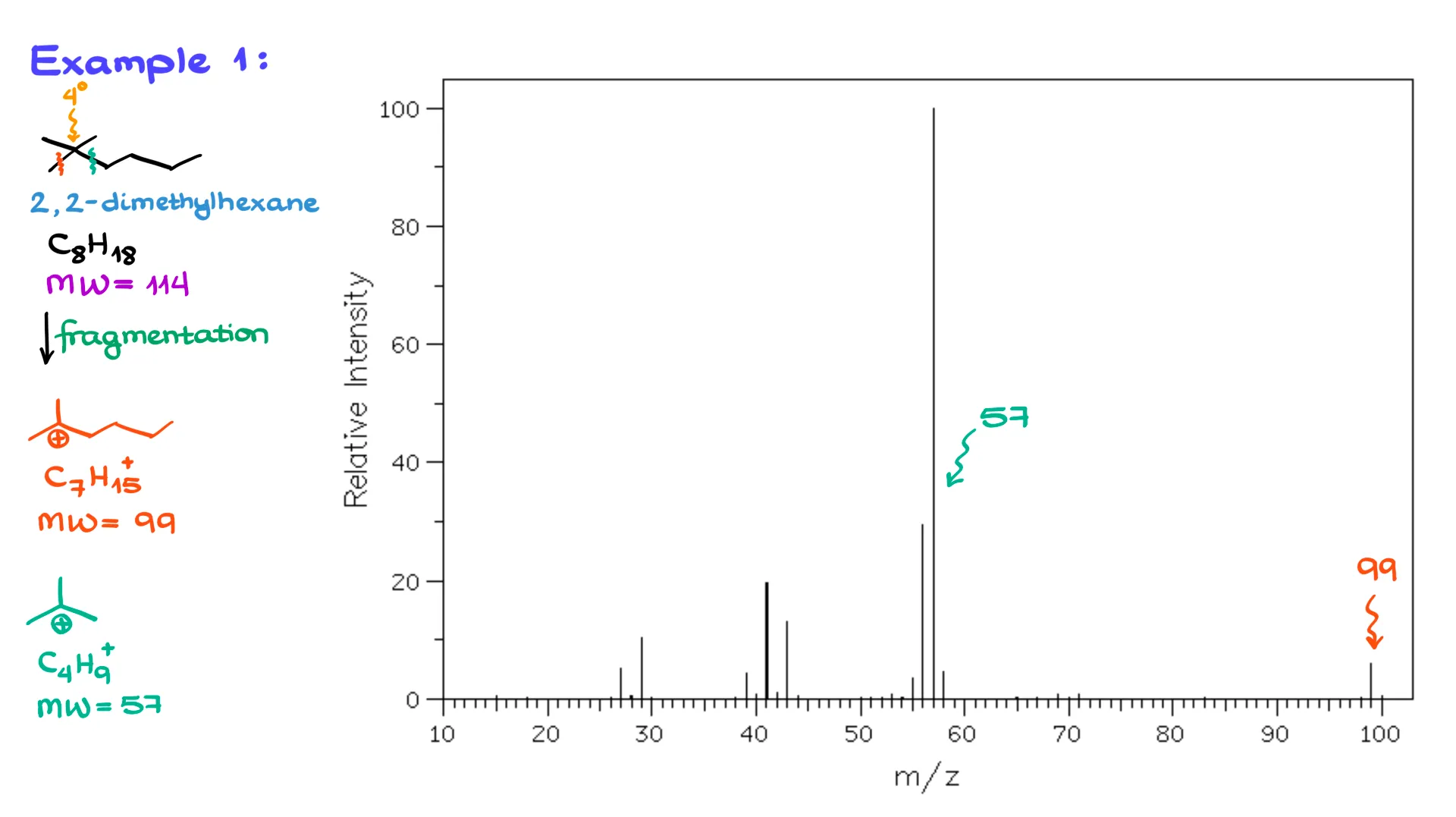
The first molecule I have here is 2,2-dimethylhexane. This molecule has the molecular formula C₈H₁₈ and a molecular weight of 114 atomic mass units. Looking at the spectrum of this molecule on the right, we don’t see the molecular ion peak, likely because the molecule isn’t stable enough for it to be detected. So, let’s see if we can identify any other peaks on this spectrum.
The first thing that jumps out right away is the quaternary carbon right here. That tells me our fragmentations are going to happen around this carbon, since that’s where we can form a relatively stable carbocation. For the first fragmentation, I’m going to chop off a methyl group, forming a tertiary carbocation, C₇H₁₅⁺, which has a molecular weight of 99. Looking at the spectrum, there it is — the peak at 99.
Next, if we break the molecule at this position, we form another tertiary carbocation with a molecular weight of 57, and we see that peak too. Typically, within the scope of our course, we don’t focus on the smaller chunks below a mass of about 40, so all that smaller stuff over here isn’t really relevant. Instructors don’t usually ask you to identify those tiny fragments anyway.
Example 2
So, with that in mind, let’s move on to a slightly more complex example. Here I have cyclobutyl methyl ether, or 2-methoxybutane if you prefer the IUPAC name.
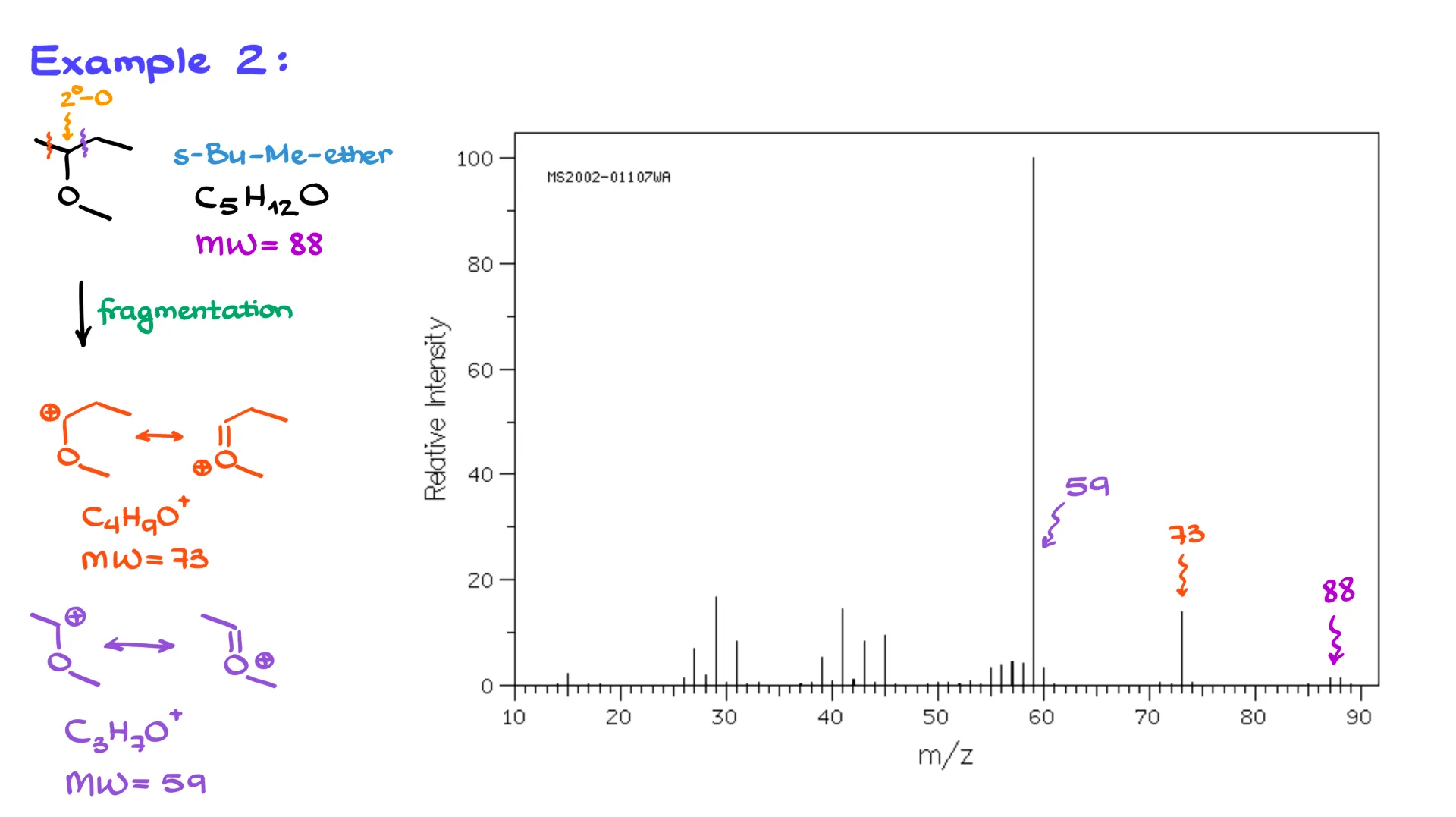
This molecule has the molecular formula C₅H₁₂O and a molecular weight of 88. And look at that, we actually have a molecular ion peak for this one. It’s small, but it’s there.
Looking at the structure, I see a secondary carbon next to an oxygen, which means the oxygen’s lone pairs can help stabilize a carbocation. So, if we do a fragmentation right here, we’ll form a resonance-stabilized carbocation with the formula C₄H₉O⁺, which has a mass of 73, and we can clearly see that signal in the spectrum. Likewise, if we cut the molecule on the other side, we form C₃H₇O⁺ with a molecular weight of 59, and that one shows up too. As before, I’m not going to pay attention to anything below about 40.
Example 3
So far, so good. Let’s move on to the next example, 3-hexanone. Its molecular formula is C₆H₁₂O, and its molecular weight is exactly 100. Looking at the mass spectrum, we can see a peak at 100, so we do detect the molecular ion.
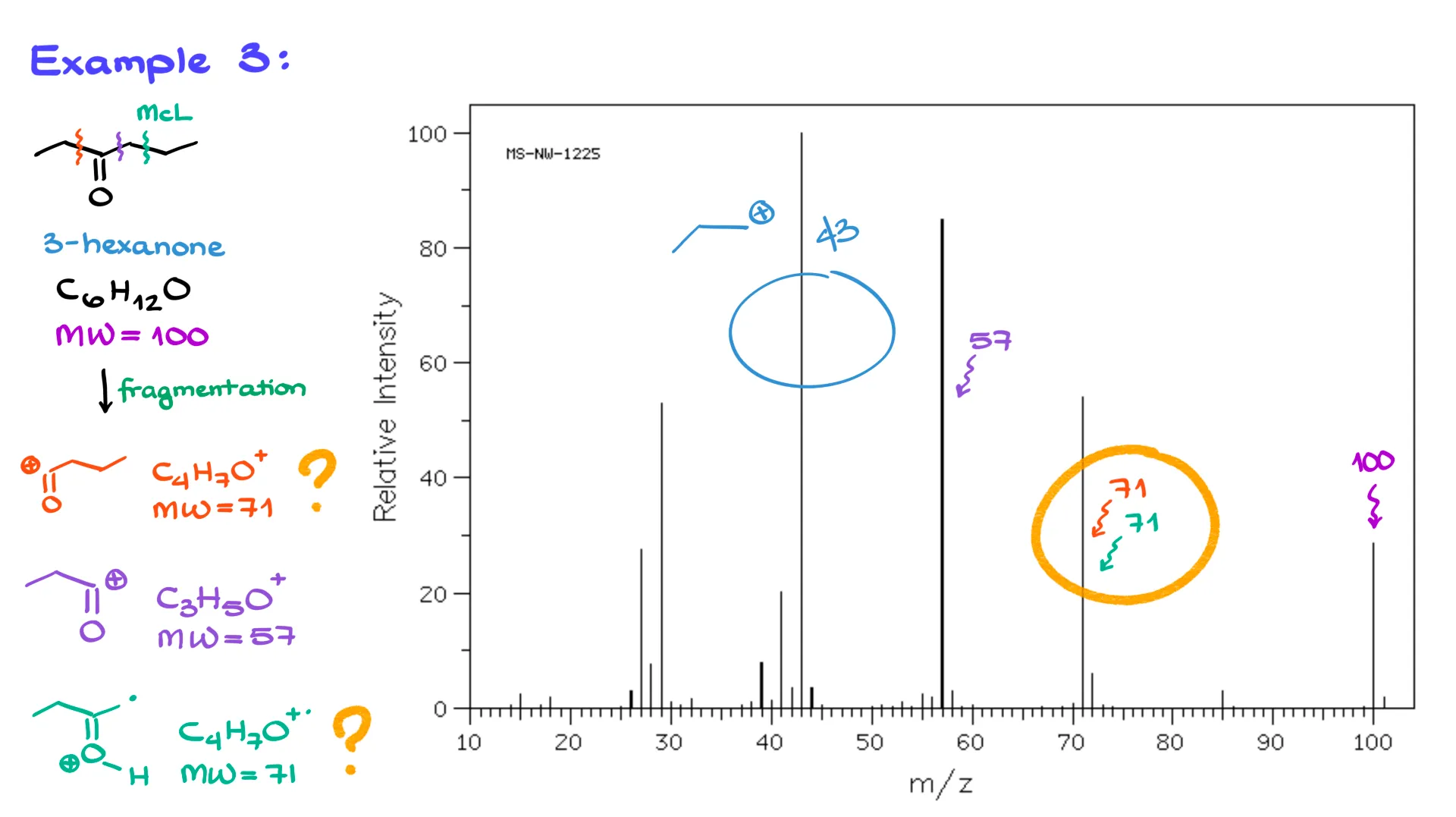
Now, this molecule has several possible places where it can fragment. The first fragmentation happens here, giving a resonance-stabilized carbocation at the carbonyl with a molecular weight of 71, and we do see that peak in the spectrum. The next possible cleavage is on the other side of the carbonyl, forming a carbocation with the formula C₃H₅O⁺, which weighs 57, and we see that one as well.
And if you’ve seen my previous tutorials, you know we can also have a McLafferty rearrangement here. That rearrangement produces a fragment at 71, which matches another signal we’re observing. There’s also a large peak at 43, that’s a propyl carbocation formed when our orange carbocation loses a CO group. Usually, we don’t focus on those smaller pieces, but it’s worth noting.
Before we move to the next example, there’s something important I want to emphasize. The biggest limitation of mass spectrometry is that it only gives you numbers — just the fragment masses. For example, that peak at 71 could come from the orange carbocation or from the McLafferty rearrangement. Unfortunately, mass spectrometry alone can’t tell us which one it is.
That’s why MS is best used as a confirmatory technique. If you already have a structure in mind, you can predict the fragments that should appear, and then check whether your spectrum shows those peaks. If it does, great, that’s good confirmation of your structure. But if you only have a spectrum and no clue about the molecule’s structure, determining it just from MS data is extremely difficult, especially for larger compounds.
Example 4
So, let’s look at a more complex case. Here we have methyl butanoate (also known as methyl butyrate). Its molecular formula is C₅H₁₀O₂ and its molecular weight is 102. Do we see the molecular ion? Yes! That tiny little peak on the far right is it.
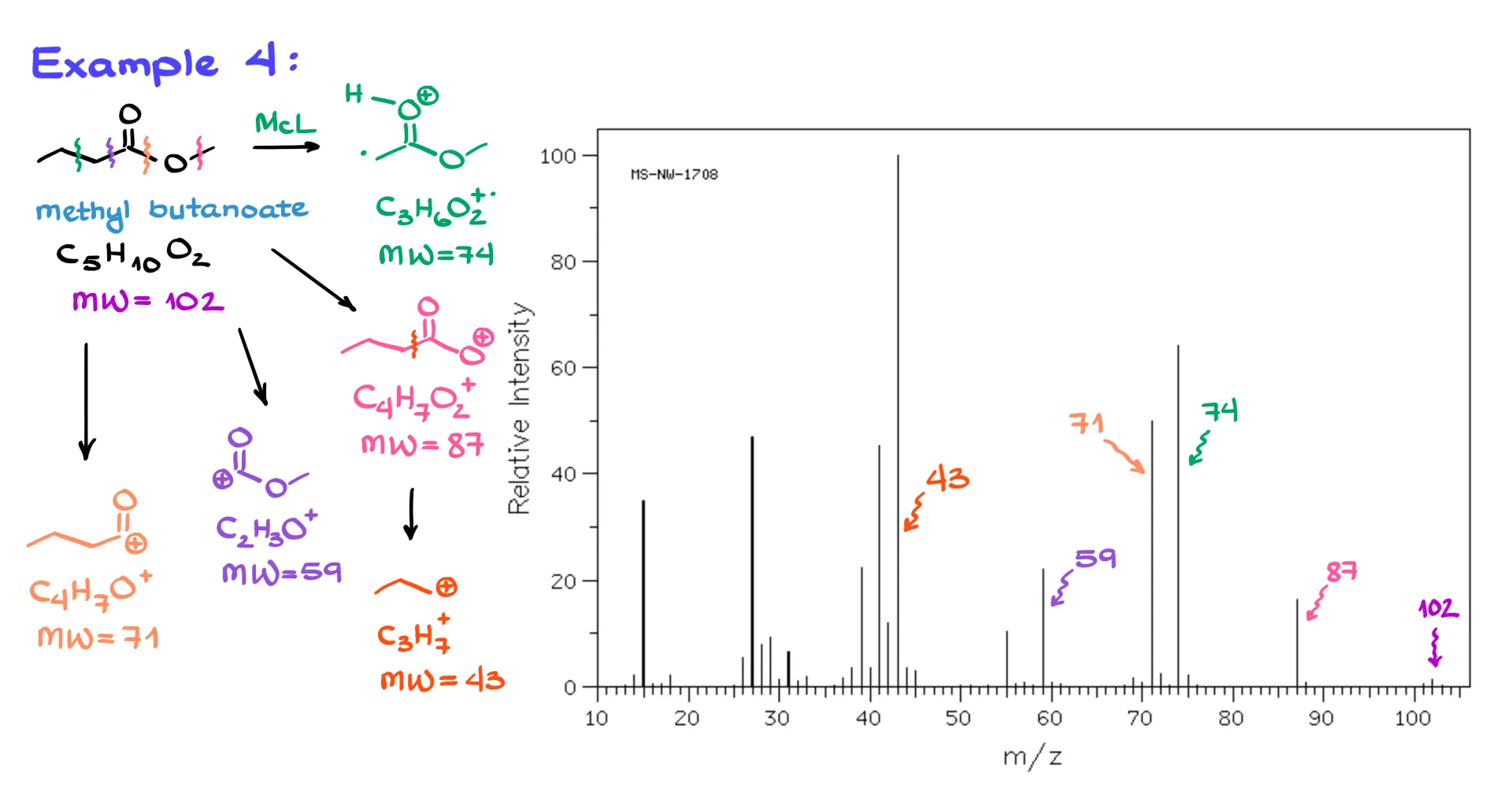
This molecule has several possible fragmentations. The first one we can identify is the McLafferty rearrangement, which gives us a fragment with a molecular weight of 74, and that peak is present in the spectrum. The next fragmentation happens when we chop off a methyl group, forming a resonance-stabilized carbocation that, while strange-looking, is still reasonably stable under mass spec conditions. That species has a molecular weight of 87, and we see that peak too.
One could argue that instead of that resonance-stabilized species, we’re just losing a methyl group from the end of the molecule, which is a valid argument and it does happen sometimes. But experimental data suggests that esters often form this exotic carbocation.
Now, are there any other fragmentations? Absolutely. We can lose a CO₂ group, forming a propyl carbocation with a molecular weight of 43, and sure enough, there’s the signal at 43. Then we can have another fragmentation that forms a resonance-stabilized carbocation at 59, we see that one too. Finally, if we chop off the methoxy group, we form an acylium ion with a mass of 71, and that signal is also visible.
There are a few smaller peaks as well: one at 55, another at 15 (that’s just a methyl cation after CO₂ loss), and one at 26. As a practice exercise, you can try to come up with reasonable fragment structures for those and see what makes sense.
Of course, there are plenty of other unique rearrangements and fragmentations we can see in mass spectrometry: things like retro-Diels–Alder reactions or two-carbon losses from aromatic systems. There’s quite a few out there. But within the scope of a typical introductory organic chemistry course, the most important ones to master are those that form the most stable carbocations and the McLafferty rearrangement.
So, if you get comfortable predicting those, you’ll be able to handle most fragmentation problems you’ll see on exams or in your homework.
Reference: SDBS : https://sdbs.db.aist.go.jp/ , National Institute of Advanced Industrial Science and Technology, date of access: 10-11-25
