What is the Difference Between a Transition State and an Intermediate?
Understanding the difference between the transition state and an intermediate will help you in drawing the mechanisms, explaining the mechanistic differences, and understanding what exactly is going on in the reaction.
So, let’s start by looking at the picture that, I’m sure, you have seen quite a few times by now:
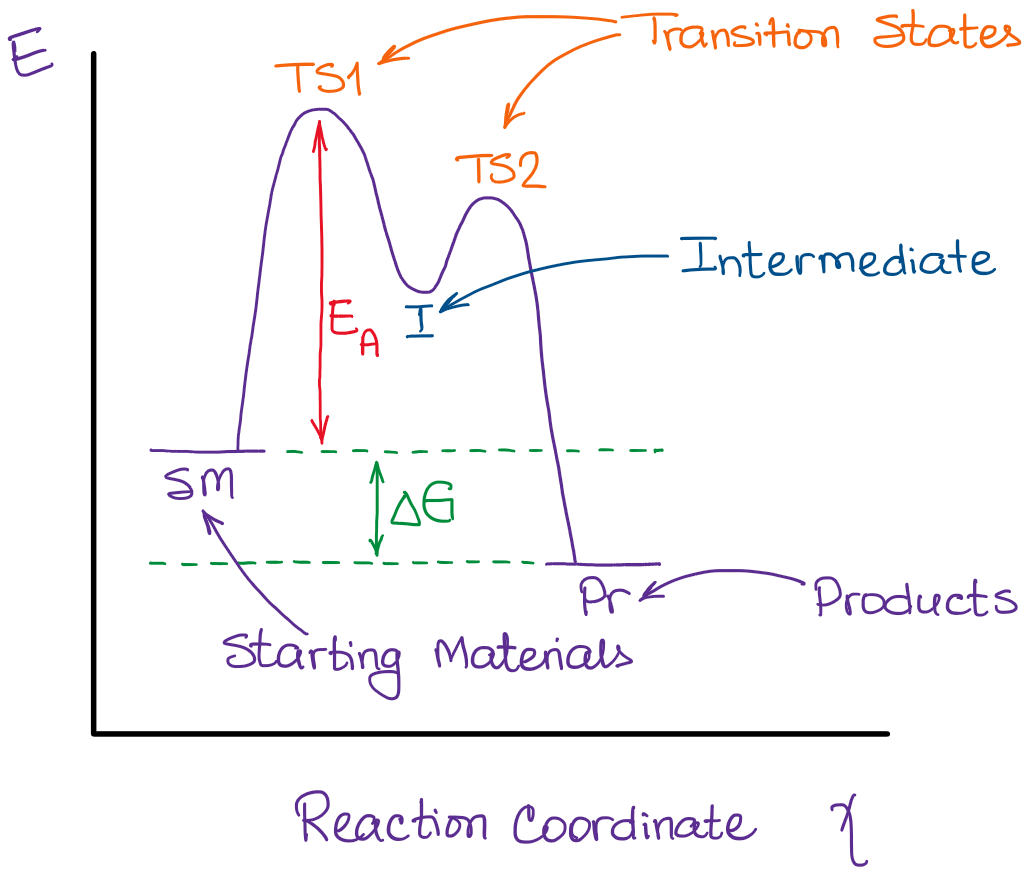
On this diagram we see:
- the x-axis that is a reaction coordinate: a loosely defined term meaning the reaction progress in the general direction from the starting materials or reagents (SM) to the products (Pr).
- the energy curve describing the energy states of the components at a certain point in the reaction.
- the transition states (TS) and an intermediate (I).
Among other things that you typically see in these diagrams are the change in energy and the activation energy, which are not too important for us at the moment. Let’s focus on the curve itself for right now. I’ve pointed out the transition states and the intermediate there, but what are those exactly?
Definition of a Transition State
Transition state is the highest point (or points) on the reaction coordinate diagram. Those are the “peaks” or the “hills” in the picture. A more strict definition is that a transition state is a molecular entity that has a lifetime no longer than a vibration that exhibits some structural characteristics of both the reactants and the products. As I couldn’t find the “official” definition, so I’ve adapted this one from the Anslyn & Dougherty’s Modern Physical Organic Chemistry book. I think, it describes the transition state the best:
- it’s neither the reactant nor it is a product
- it resembles both to some extent, and
- it’s not something that can be isolated (exists only a vibration-long)
The best analogy I can think to describe a transition state is this: imagine yourself merrily hopping down the alley in a park. That moment in time, when you’re up in the air in the mid-jump is your transition state! You cannot “catch” that state when you’re suspended in the midair, it’s neither your right leg, nor it is your left leg step… it’s something in between. Same applies to transition states: they are somewhere in between.
Definition of an Intermediate
Intermediate is a comparatively long-lived species that can be experimentally detected and characterized. This means that an intermediate is an actual molecule or an ion that you can work with, sometimes even isolate. That’s you freezing in space as you’ve landed on one foot while hopping in a park. It’s not the beginning of your journey (reactants), nor it is the end (products). Instead, it’s a relatively stable midpoint (intermediate).
It is also very important to remember that a reaction doesn’t have to have an intermediate! You may or may not have one depending on the nature and the mechanism of the reaction. There are plenty of single-step reactions that have no intermediates at all. A typical first semester organic chemistry example is an SN2 reaction that involves no intermediate whatsoever (we’ll see an example later in this post).
You will always have a transition state though! Every reaction, no matter how simple it might be, has a transition state. For instance, let’s consider the following bromine dissociation reaction giving us two bromine radicals:
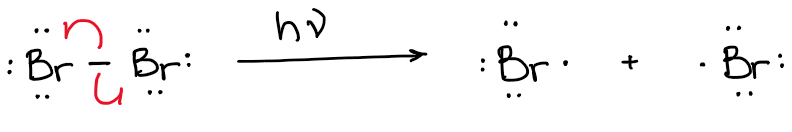
The two bromine atoms didn’t just magically split and appeared in different places in space. They first had to stretch the bond to the brink of breaking (explaining it very simplistically here) and then break apart:
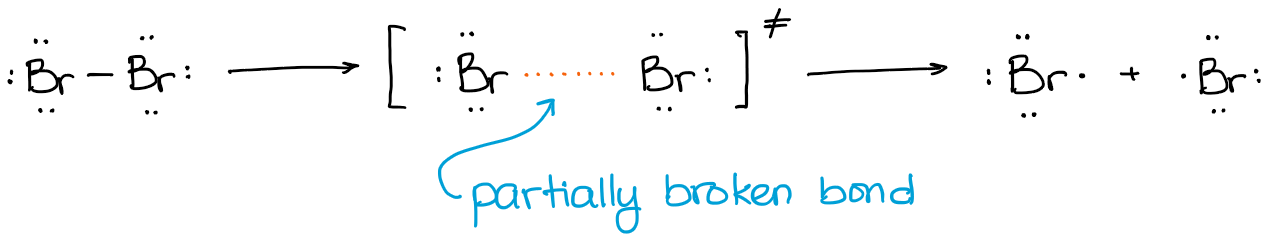
So, the transition state in this reaction is an elongated bond which is almost, but not quite, broken.
In a nutshell, you’ll have a transition state for every single step in your reaction. So, you can always check the number of transition states by counting the steps in the mechanism and vice-versa.
Example of a Reaction with One Transition State and No Intermediates
Reaction above was an example of a reaction with no intermediate. Let’s look at another one:
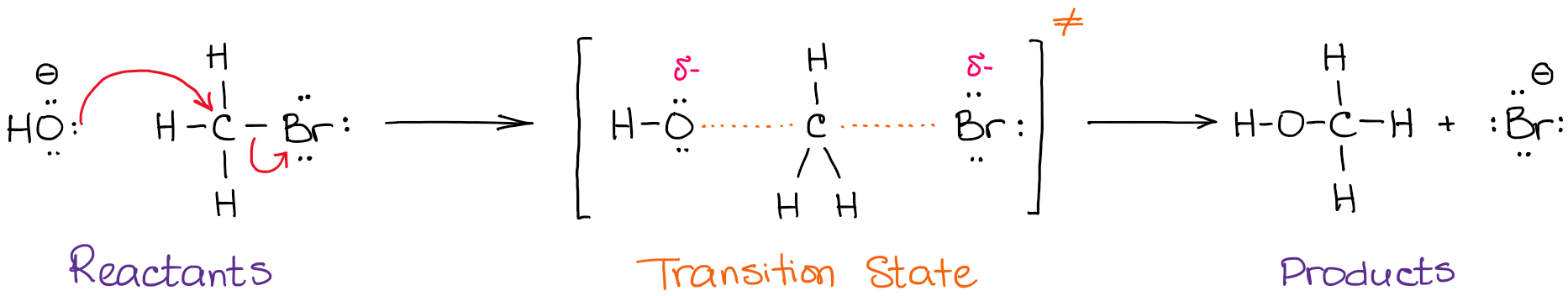
This reaction is an example of an SN2 reaction where no intermediate is formed. You do have a transition state though in which the O-C bond is not quite formed yet and the C-Br bond is not quite broken. The energy diagram for this reaction will look like this:
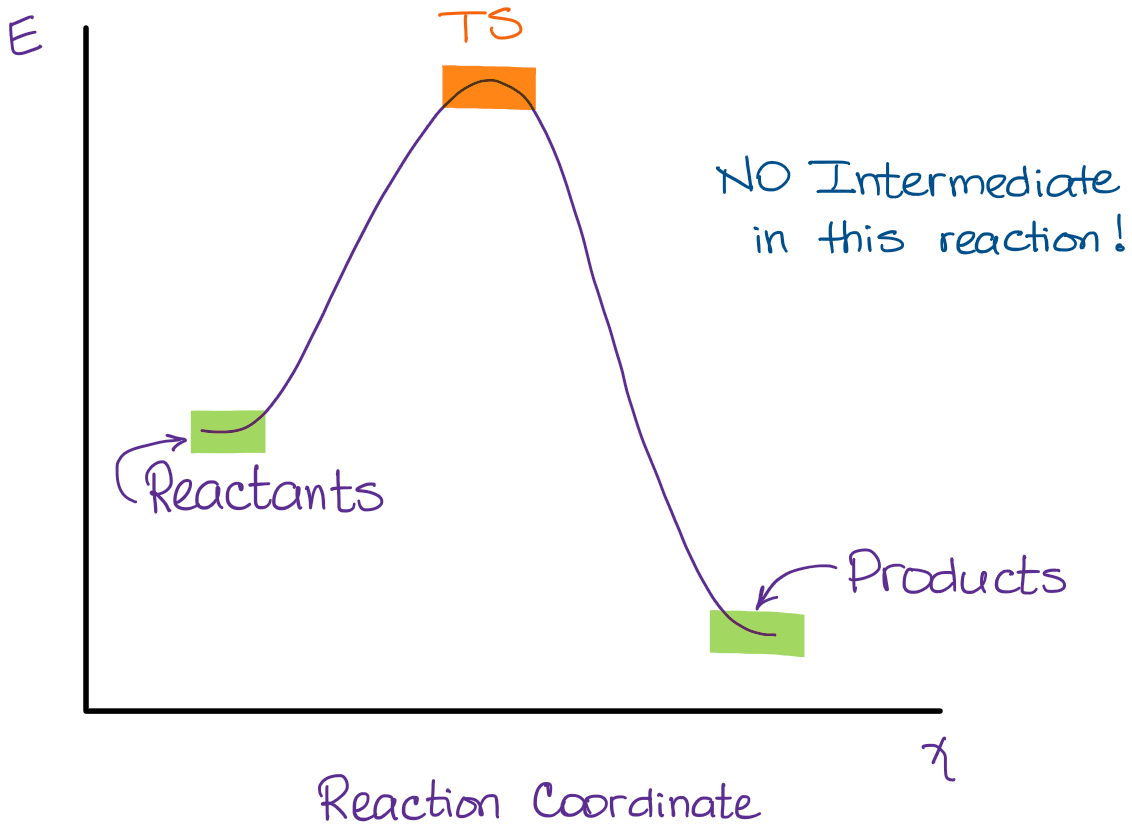
So, we “hop” into the air, and we land with new products in hand and no stops in the “mid-air” for an intermediate. How does a reaction with an intermediate look like then?
Example of a Reaction with Two Transition States and One Intermediate
Well, since we’re already talking about the substitution and elimination reactions, the E1 mechanism is a perfect example of such a reaction:
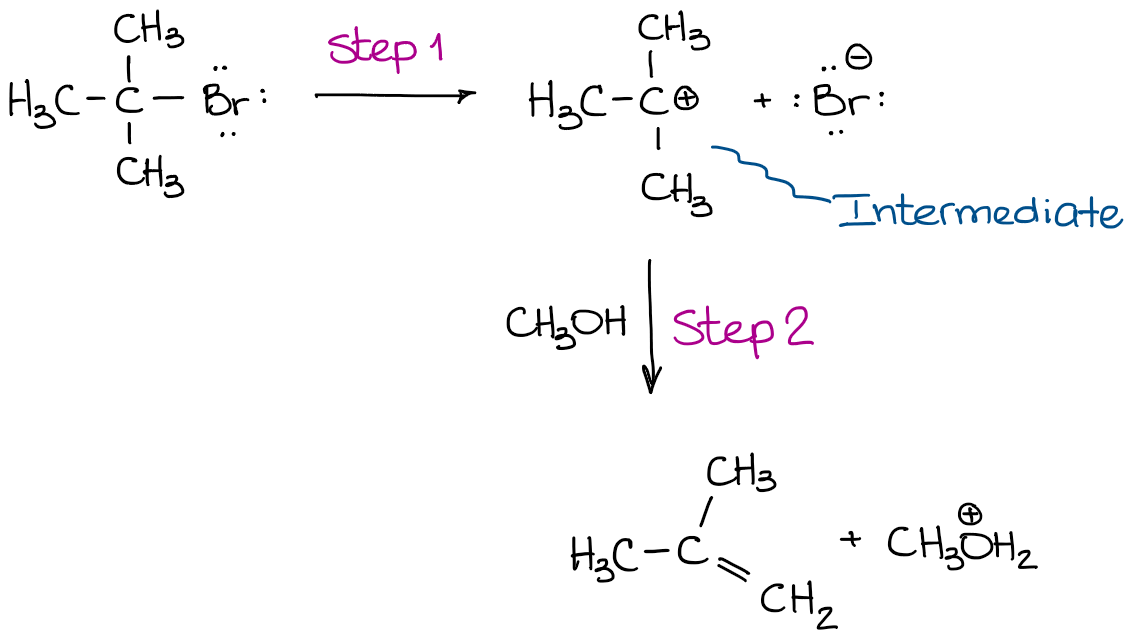
In the reaction above, an intermediate is formed. That intermediate is produced before the reaction proceeds into the next step. And it is the intermediate that reacts further giving you the final product. So, if we analyze it step-by-step, we’ll get the step 1:
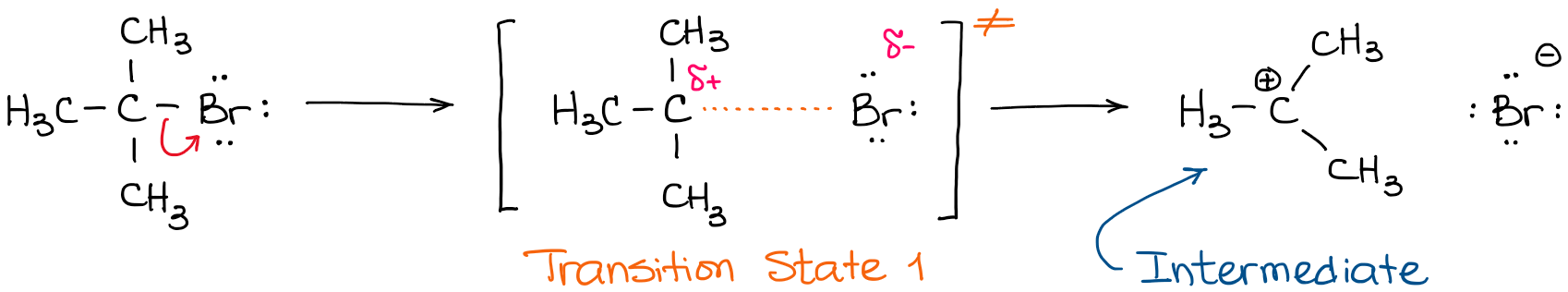
The first transition state is the process of the C-Br bond elongation that leads to the eventual bond dissociation and the formation of the carbocationic intermediate. This intermediate does exist long enough (although, still very briefly) so that we can “catch” it using experimental techniques and characterize it. The second step in this reaction has its own transition state:
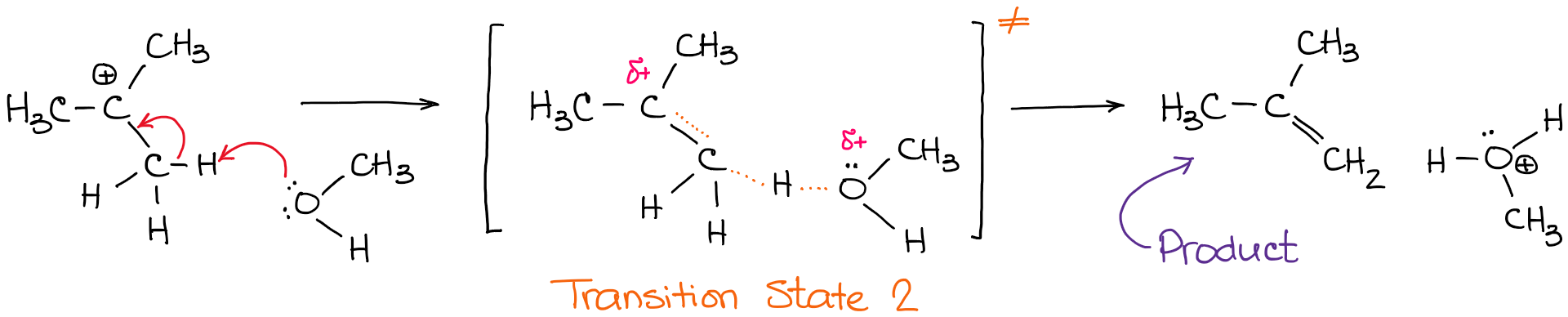
In the second step above we have three different bonds in the “making-breaking” state. The H-O and C=C bonds are forming, while the C-H bond is breaking. Now, if I were to plot this reaction on the reaction coordinate vs energy plot, we’ll get something like this:
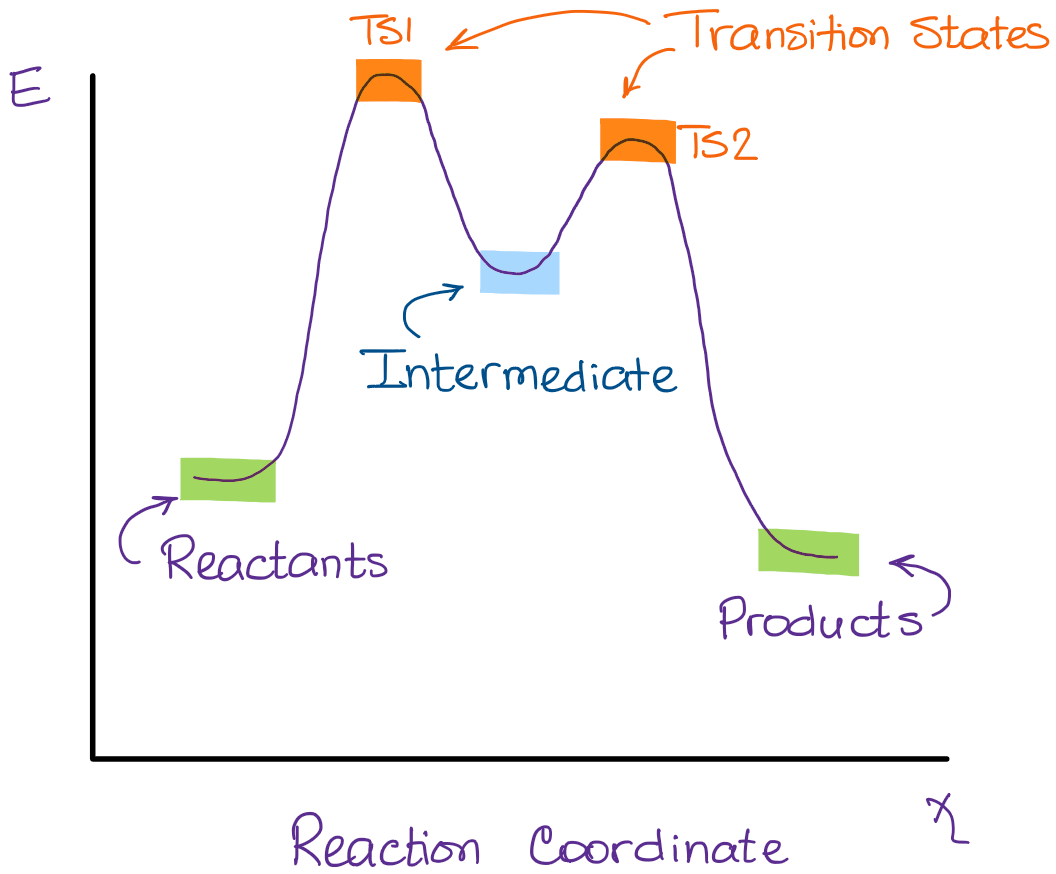
A quick rule-of-thumb for identifying the transition states and intermediates in the reaction is to look for the hilltops and valles on the diagram. Your hilltops are the transition states, your valles are the intermediates. The beginning of the curve is the reactants and the end is your products.
Remember, your reaction may have a very long mechanism! Reactions with 5- or even 10-step mechanisms are not as rare as you might think. We’re generally not going to look at anything like that in the first semester organic chemistry, but brace yourself for page-long mechanisms mid-second semester!
When we draw the mechanisms, we typically never show the transition states unless it is essential to explain an observation or the reaction behavior. For instance, it’s impossible to explain the “endo rule” in the Diels-Alder Reaction without considering the transition states.
What to Expect on the Exam?
There are 3 common types of a question you’re going to see on the test that deal with transition states.
Type 1 is a question that shows you a reaction diagram and asks for the transition states and intermediates. For instance:
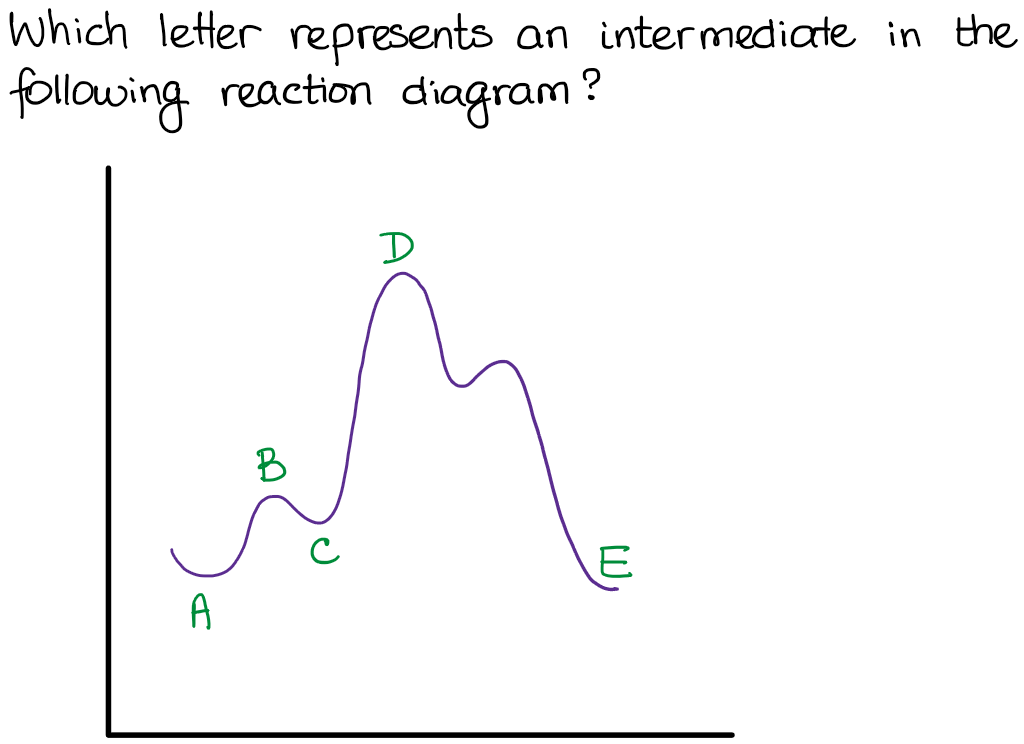
As you remember, the intermediate is the “valley” in the diagram, so it must be the option C in this case.
Type 2 is to count the number of intermediates, transitions states, or the mechanistic steps in the reaction based on the diagram. The reaction diagram above has 2 intermediates and 3 transition states, so it is a 3-step reaction.
Finally, the last question you can expect is a question about the shape or a nature of the transition state itself. We know that the transition state is something in-between the reagents and products/intermediate. To estimate the exact nature of the transition state, though, we’ll need to use a principle known as Hammond’s postulate, which is a whole topic on it’s own, and I will talk about some other time.

Ascorbic acid is a really important vitamin (obviously) that degrades/ oxidizes in water, in the presence of trace metals. think copper cooking pots. currently, there are two competing rate equations (with/ without a term for oxygen (like so)
rate = k. [metal] [ascorbate] [O2]
this is followed by competing narrative about “intermediates” states/ transition states (which I found confusing/irritating). some researchers had detected short-lived (ascorbate-metal) complexes…etc. BUT i recall vaguely from my from college organic chem. course (taken 30 years ago!) that (#1) rate equations usually indicate which entities are involved in a transition state, (#2) transition states cannot be isolated. a google search “can you isolate a transition state” brought me to this site. the content here is relevant, easy to understand and the accompanying diagrams are excellent. my only mall bit of criticism is one of accreditation. It’s taken a while to find who wrote this content and how to credit/ cite this material in my own writing. I highly commend this material for anyone needing a fresher.
Typically, website references are not considered academically “solid” types of a reference. However, if you want to reference to a website (and people often do even in peer-reviewed publications), you’d normally give the link and state when it was accessed. For instance, if you wanted to reference this page, you’d say “[1] https://www.organicchemistrytutor.com/topic/what-is-the-difference-between-a-transition-state-and-an-intermediate/, accessed Feb 13, 2023″ or something of the sort depending on the publication reference style.
thank You Soo much 🥰🥰🥰…this is exactly what I was looking for. …