Typical IR Exam Questions
In this tutorial, I’m going to walk you through the most common types of IR exam questions and how to approach them effectively.
Example 1
Let’s jump into the first set. These types of questions are popular with instructors who like open-ended formats. Here, we’re asked to describe the main features of each molecule that we could identify using IR spectroscopy. To do that, you need to make sure you know your functional groups!
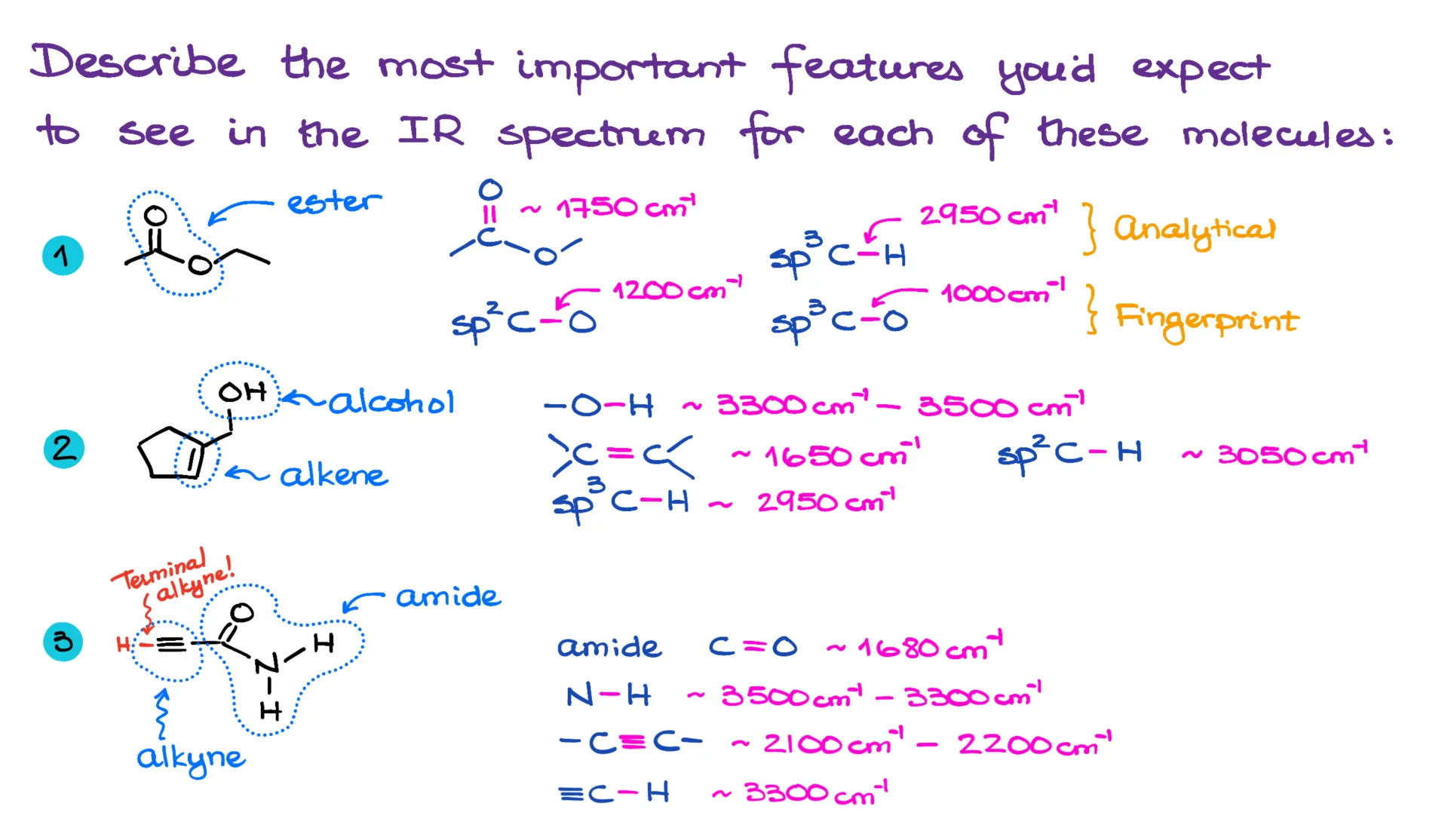
Take the first molecule, for example—this one’s an ester. And because it’s an ester, we’ll expect to see certain features. Namely, a C=O stretch at about 1750 cm⁻¹. We should also see the regular sp³-hybridized C-H stretch around 2950 cm⁻¹, which leans onto the 3000 line from the right. In the fingerprint region, we’d expect the sp² and sp³ C-O stretches near 1200 and 1000 cm⁻¹, respectively. But like I’ve said before, unless you really know how to read the fingerprint region, it’s best to focus on the analytical part of the spectrum. Other than that, we don’t expect anything particularly distinctive from this molecule’s IR spectrum.
Now, the next molecule is an alcohol. That means we’re going to be looking for a strong, broad, and smooth signal somewhere around 3300–3500 cm⁻¹. This molecule also has an alkene, so we should see a C=C stretch near 1650 cm⁻¹, and an sp²-hybridized C-H stretch just above 3000. Naturally, we’ll also spot the sp³-hybridized C-H stretches around 2950. While that last one is super common and shows up in most organic molecules, it’s still worth noting since it lies in the analytical region of the IR spectrum—and that’s our main focus.
My next molecule is an amide, which gives us a few distinctive signals. First off, there’s the amide carbonyl, which typically shows up at a slightly lower wavenumber than a regular carbonyl—somewhere around 1680 cm⁻¹ instead of the usual 1720–1750. We also expect to see an N-H stretch in the general 3300–3500 cm⁻¹ region. This one’s going to be broad too, but not as strong or smooth as an alcohol’s -OH stretch, so we can usually tell them apart by the shape.
In addition to the amide, this molecule also contains an alkyne. That means we’ll likely see the C≡C vibration around 2100–2200 cm⁻¹. And because it’s a terminal alkyne, we’ll also see a sharp spike at almost exactly 3300 cm⁻¹ from the sp-hybridized C-H stretch. That signal could overlap with the amide’s N-H and create a bit of a mess in that region. So, the key takeaway for these types of questions is this: know your functional groups, and know the IR features associated with their bonds.
Example 2
Next up is a similar kind of question where we’re given a pair of molecules and asked how to distinguish them using IR spectroscopy. In this case, you want to zero in on the differences between the functional groups. I like to make a quick checklist of all the expected signals in the analytical region for each compound.
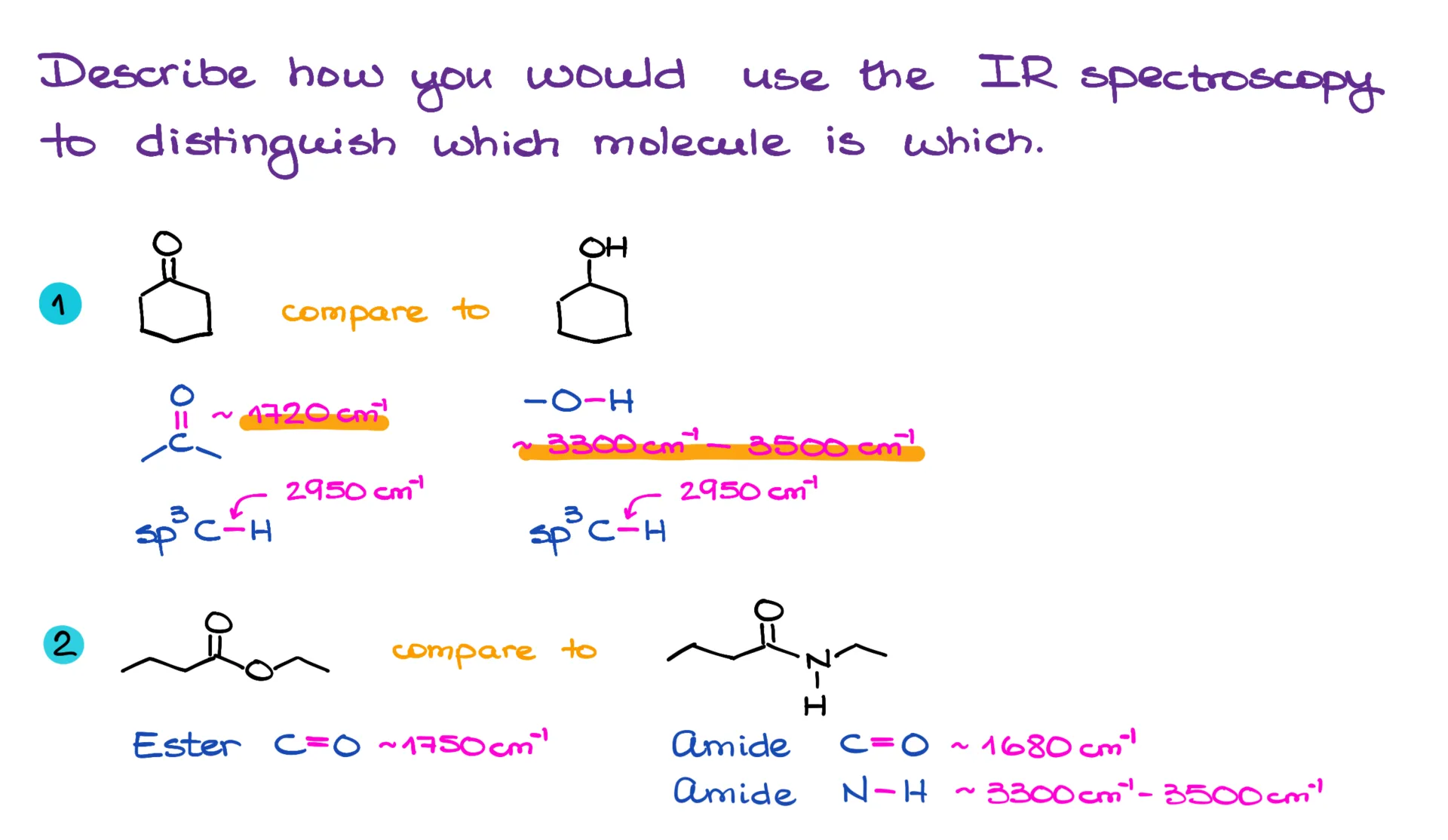
For instance, with cyclohexanone on the left, we’d expect to see a carbonyl stretch and the regular C-H stretches. For cyclohexanol on the right, we’d see the broad -OH stretch instead of the carbonyl, and of course, the usual C-H stretches. So, the distinguishing feature here would be the presence of a sharp spike around 1700 cm⁻¹ for the carbonyl in one molecule, versus a broad, smooth signal around 3500 cm⁻¹ for the -OH in the other.
In another example, we might be comparing an ester to an amide. The ester’s carbonyl typically shows up around 1750 cm⁻¹, while the amide carbonyl appears closer to 1680 cm⁻¹. That 70 cm⁻¹ difference is actually pretty significant—IR is a precise technique, and shifts of 20 cm⁻¹ can matter a lot. On top of that, the amide will also give you a broad N-H stretch between 3300–3500 cm⁻¹.
Now, your instructor might wrap these questions in all sorts of creative ways. Maybe you’re told a student mislabeled reagent bottles, or you’re asked how to use IR to monitor a reaction. No matter the story, these questions always come down to comparing expected IR signals from one compound to another.
Example 3
Now comes the real fun—matching compounds to spectra. These are usually multiple-choice questions. You might get one spectrum and several possible molecules, or one molecule and a few spectra, or even a batch of both. Regardless of the setup, the strategy stays the same. First, look at your molecules. Then determine the functional groups. Jot down the expected signals in the analytical region, and compare them to your spectra.
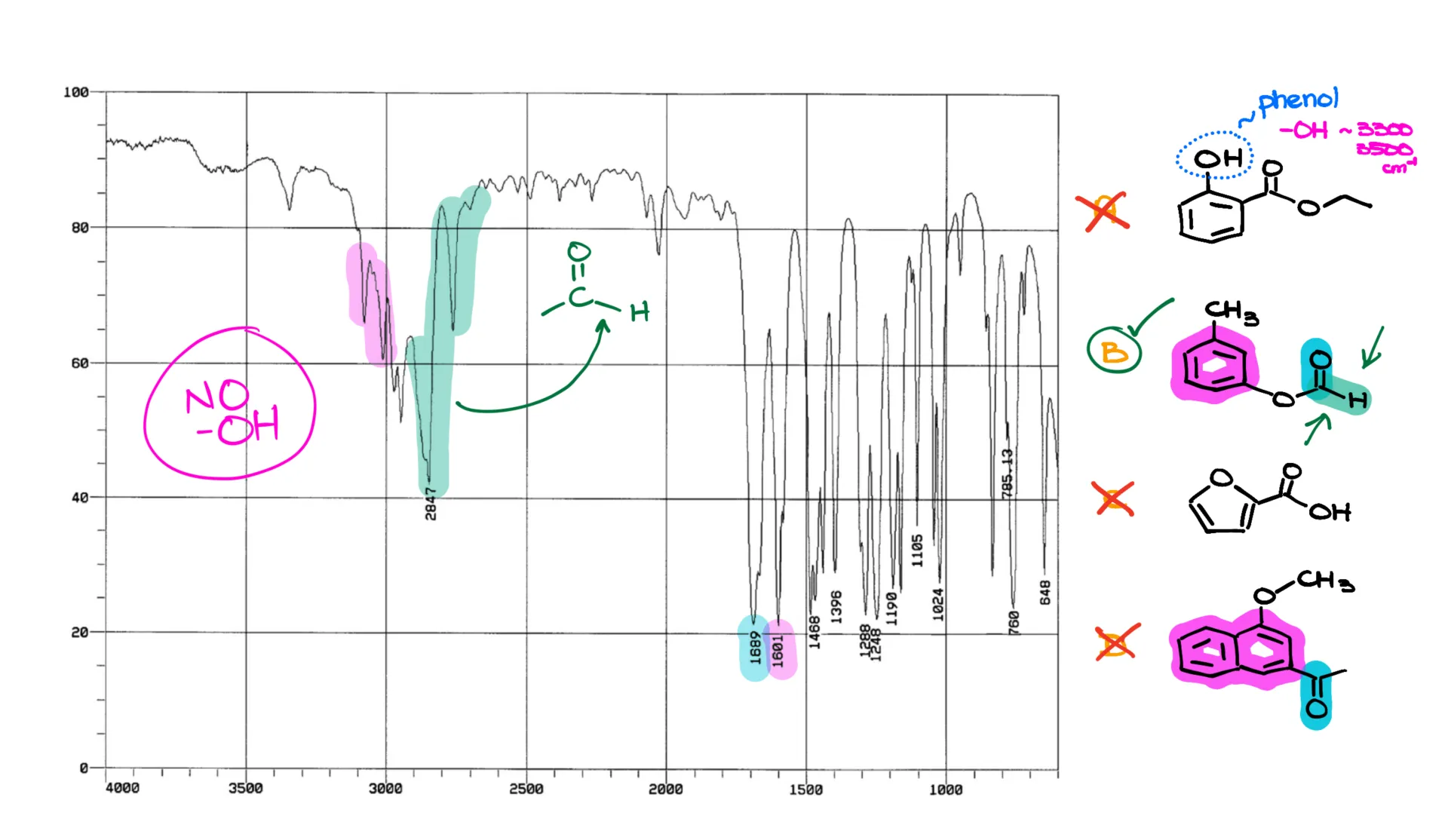
Here’s an example. My first molecule is a phenol, technically an alcohol with an aromatic ring. It should give a broad signal around 3300–3500 cm⁻¹. But I don’t see anything like that in this IR spectrum. So, compound “A” is out. For the same reason, compound “C”—a carboxylic acid—is also out.
Now, compounds “B” and “D” both have carbonyls, which we see at around 1700 cm⁻¹. Both are also aromatic, and we see that reflected in the 1600 cm⁻¹ region from aromatic C-C stretches, and those signals just above 3000 cm⁻¹ from the sp²-hybridized C-H stretches. So, how do we tell them apart? At first glance, it might seem impossible.
But remember what I said earlier—spectroscopy is a bit of an art. With practice, you learn to spot the subtle details. In this case, a trained eye would notice the Fermi doublet—a subtle feature that looks like two “vampire fang” peaks. One of those overlaps with the C-H stretch and extends a bit too far. That’s a classic sign of an aldehyde-style C-H stretch.
And look at that—one of our molecules has exactly that feature. Bingo!
So you see, practice is everything. And yes, I picked this tricky example on purpose. A student who’s worked through a few dozen problems would likely spot this without much trouble. But if you’ve only read the textbook and haven’t done much practice, you’d probably miss it—and then complain the question was unfair.
I’ve seen thousands of student tests and written hundreds myself as an instructor. If you thought this question was hard, then you probably need more practice. It’s a fair question, and honestly, just the right level of difficulty for a typical organic chemistry class. Use this as a way to check your current skills in IR.
So, if this were your spectroscopy quiz today—would you pass? Or do you think you need a bit more work to sharpen your skills? Let me know in the comments. I love reading them!
