PCC (Pyridinium Chlorochromate)
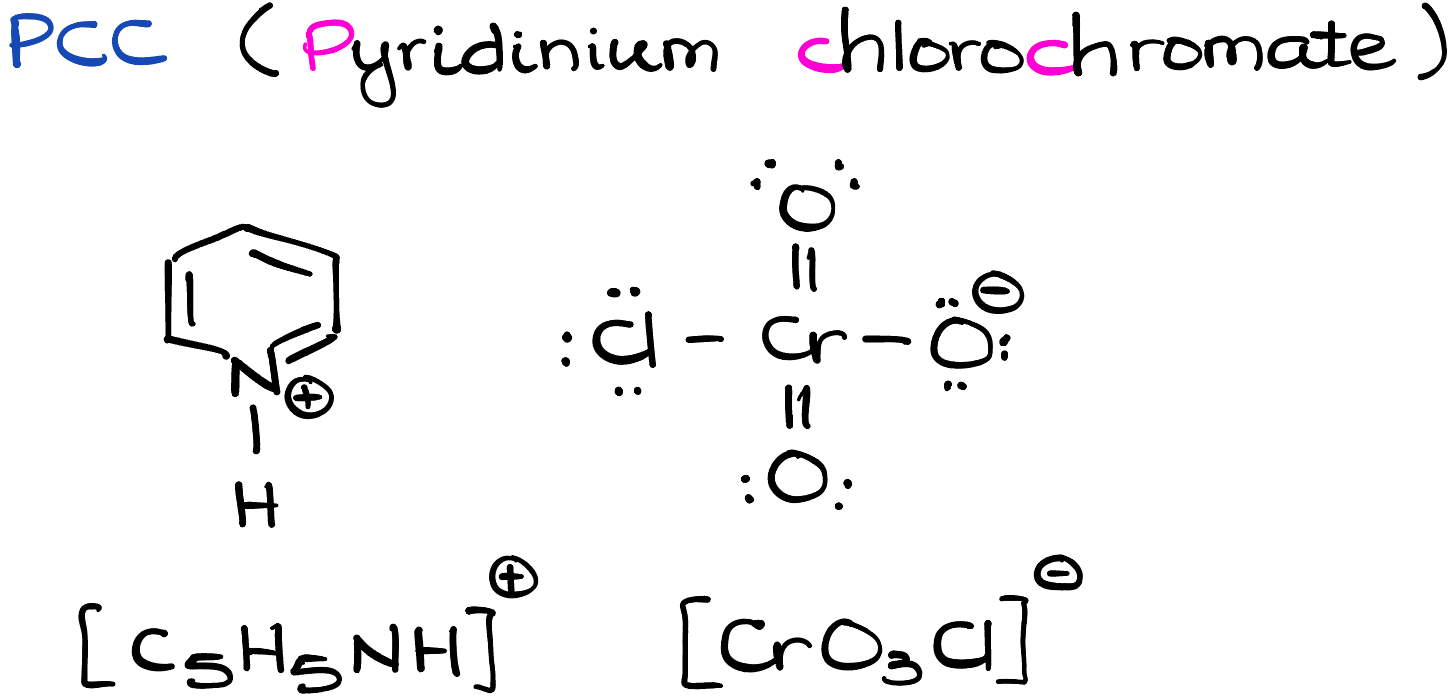
Introduction
When it comes to oxidative transformations in organic chemistry, pyridinium chlorochromate (PCC) is a name that frequently arises. As an incredibly powerful oxidizing agent, PCC has etched its place in synthetic chemistry and finds diverse applications in laboratories worldwide. It facilitates several crucial reactions that help transform simple substances into complex molecules. In this entry, we will delve into the chemical properties of PCC, highlighting its significance in the world of organic chemistry.
Chemical Properties
Pyridinium chlorochromate, C5H5NH+ ClCrO3-, is an orange-red, crystalline solid. Its main chemical identity is tied to its strong oxidizing properties. Structurally, PCC consists of a positively charged pyridinium ion (C5H5NH+) and a negatively charged chlorochromate ion (ClCrO3-).
The true power of PCC lies in the chlorochromate ion. Chromium, situated in the middle of the Periodic Table, has multiple oxidation states, making it a suitable candidate for redox reactions. In PCC, chromium is in the +6 oxidation state, which is its highest oxidation state for this element. As such, it is eager to gain electrons, making PCC a potent oxidizing agent.
One of the notable properties of PCC is its selective oxidation. Unlike some other common oxidants, PCC can oxidize primary alcohols to aldehydes and secondary alcohols to ketones without over-oxidizing to carboxylic acids or breaking carbon-carbon bonds. This remarkable selectivity makes PCC a preferred reagent for delicate oxidation tasks, particularly when a high degree of control is necessary.
Significance in Organic Chemistry
PCC has become an essential tool in organic synthesis due to its reliable and selective oxidizing power. Its ability to gently oxidize primary alcohols to aldehydes and secondary alcohols to ketones is particularly valuable, as these reactions are often difficult to control. This selectivity is due to the absence of water in PCC reactions, which prevents the further oxidation of aldehydes to carboxylic acids. Sometimes PCC is called a “weak” oxidizing agent because it doesn’t overoxidize alcohols. However, this is an incorrect “white lie” that is often taught to organic chemistry students. PCC is a pretty strong oxidizing reagent and the trick to avoid the overoxidation is in keeping your reaction absolutely dry, aka without any water present. In the presence of water, PCC will readily oxidize primary alcohols to carboxylic acids.
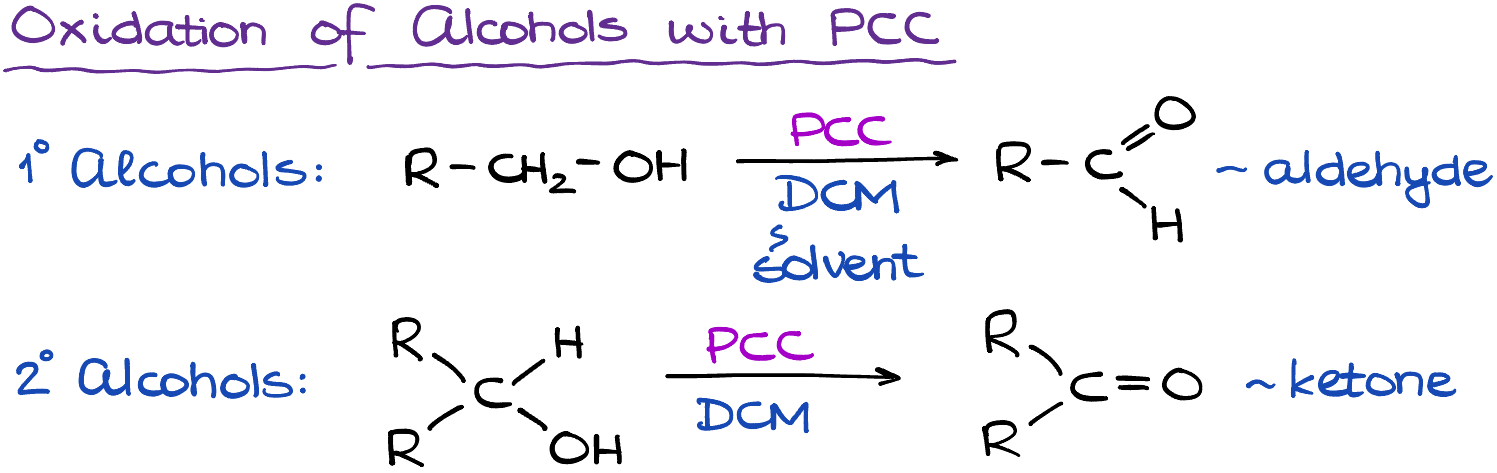
PCC is commonly used in conjunction with a polar aprotic solvent dichloromethane (DCM). As DCM is a good organic solvent with low boiling point and excellent ability to dissolve many organic substances, it’s a good choice for the reactions involving PCC.
Moreover, PCC can perform these oxidations under mild conditions, without necessitating high temperatures or harsh acidic environments. Therefore, it is possible to conduct these reactions in the presence of other functional groups that might be sensitive to harsh conditions.
Safety Information
Like many powerful reagents in chemistry, PCC must be handled with care. It is a strong oxidizer and can cause fires if it comes into contact with organic material. Therefore, it is important to store PCC away from combustible materials.
In terms of health hazards, PCC is toxic if inhaled or swallowed, and it can cause severe skin burns and eye damage. As with all hazardous chemicals, appropriate personal protective equipment, including gloves, safety glasses, and lab coats, should be worn when handling PCC.
In the event of a spill, PCC should be cleaned up while avoiding dust formation. It should not be released into the environment due to its harmful effects on aquatic life. Always dispose of PCC responsibly, following local waste disposal regulations.
Due to the chromium toxicity, PCC doesn’t see much use nowadays. The use of halogenated solvents like DCM is another environmental concern that we need to consider when weighing whether to use PCC or opt for another reagent to accomplish the oxidation reaction you need.
