31. Synthesis of a Complex Enone from a Ketoacid
In this tutorial, I want to walk through a fun-looking synthesis where we start with 4-oxapentanoic acid and end up with a cyclic enone as our final product.

So, when we’re looking at the final product and the answer doesn’t jump out right away—when we don’t see a clear strategy—we turn to retrosynthetic analysis. In this case, I can immediately recognize that the final product is an α,β-unsaturated compound. We typically form these kinds of molecules via aldol condensation, which means our starting material should be something that lends itself to that pathway. Looking at the final step of the synthesis, it appears that the double bond is formed between two specific carbons.
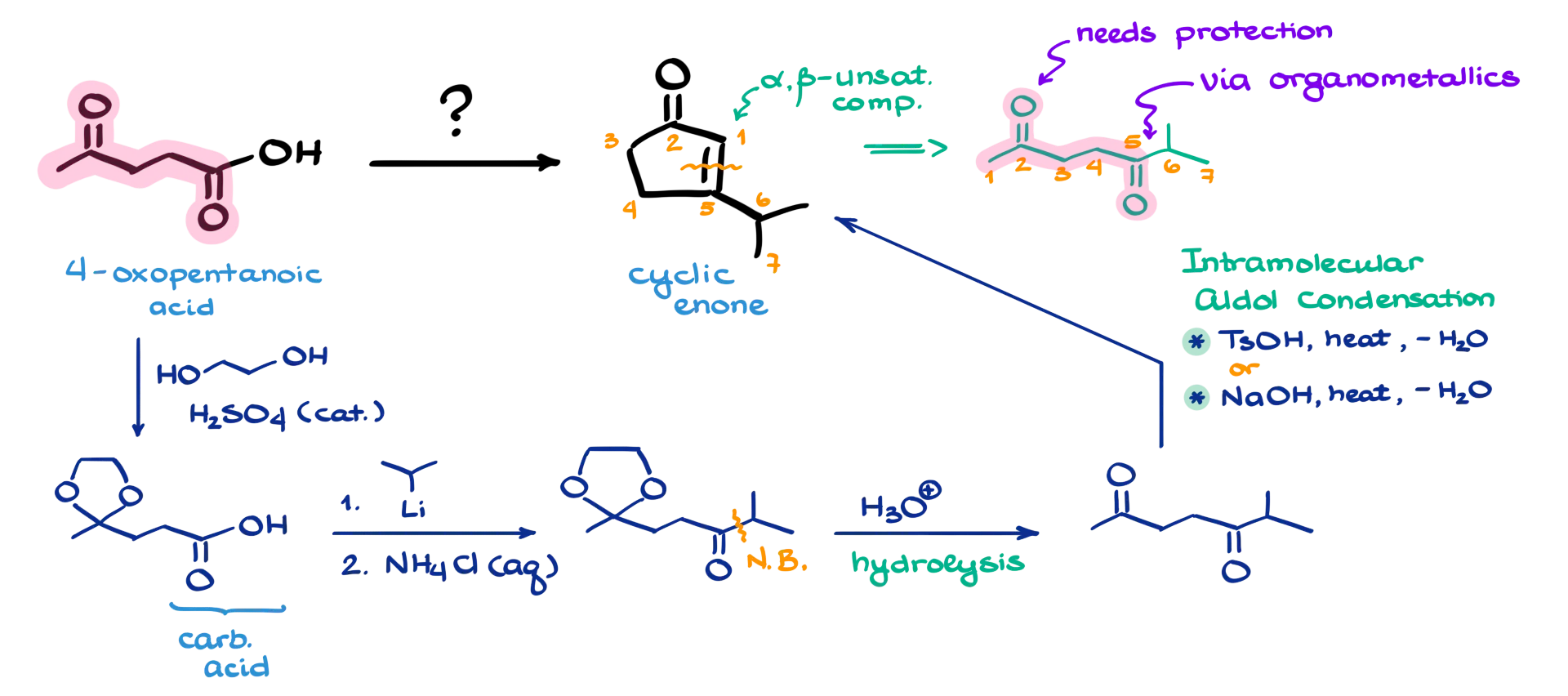
To make the mapping between the starting material and the product clearer, I’ll start by numbering the carbons in the final product. Then, I trace those same carbons in the starting material used in the aldol condensation. That helps me see where everything connects. Specifically, it looks like we’ll be forming the bond between carbons 5 and 6, most likely through a reaction with an organometallic compound. And because we’re using an organometallic reagent, the carbonyl on carbon 2 in our starting material needs to be protected.
So, I’m going to start by protecting the ketone using a simple acetal formation under acidic conditions. That gives us a protected intermediate, which allows us to proceed with the next step without unwanted side reactions. Now, we still have the carboxylic acid group, and the goal is to create a new carbon–carbon bond between the carbonyl carbon and an incoming carbon nucleophile. There are multiple strategies for that, but probably the easiest and most straightforward is using an organolithium compound.
Why organolithium? Well, when it comes to organometallic chemistry, organolithium reagents are strong enough bases to deprotonate the carboxylic acid and then perform a nucleophilic attack on the resulting carboxylate. On the other hand, Grignard reagents (organomagnesium compounds) tend to stop after the acid–base step and won’t proceed to form that new bond. If you’re curious to dive deeper into this chemistry, I’ll leave a link in the description where you can explore this in more detail.
Now that we’ve created the key carbon–carbon bond, we can remove the acetal protecting group by hydrolyzing it, which regenerates the carbonyl on carbon 2. From here, we’re set up for an intramolecular aldol condensation. There are a few ways to carry this out. One option is using acidic conditions—acid as a catalyst plus heat to drive off water. Another approach is to use basic conditions, such as sodium hydroxide or another base, to catalyze the reaction. There’s also a hybrid approach where you start in base and then switch to acidic conditions to complete the process.
Whichever method of aldol condensation you prefer, it’ll work just fine in this case. That’s how we build our final product: a cyclic enone, formed through a strategic combination of protection, organolithium addition, and aldol condensation.
Now, here’s something to consider: what if we didn’t know how organolithium compounds react with carboxylic acids? How else could we design this synthesis? What alternative route would you take? Let me know in the comments below.
