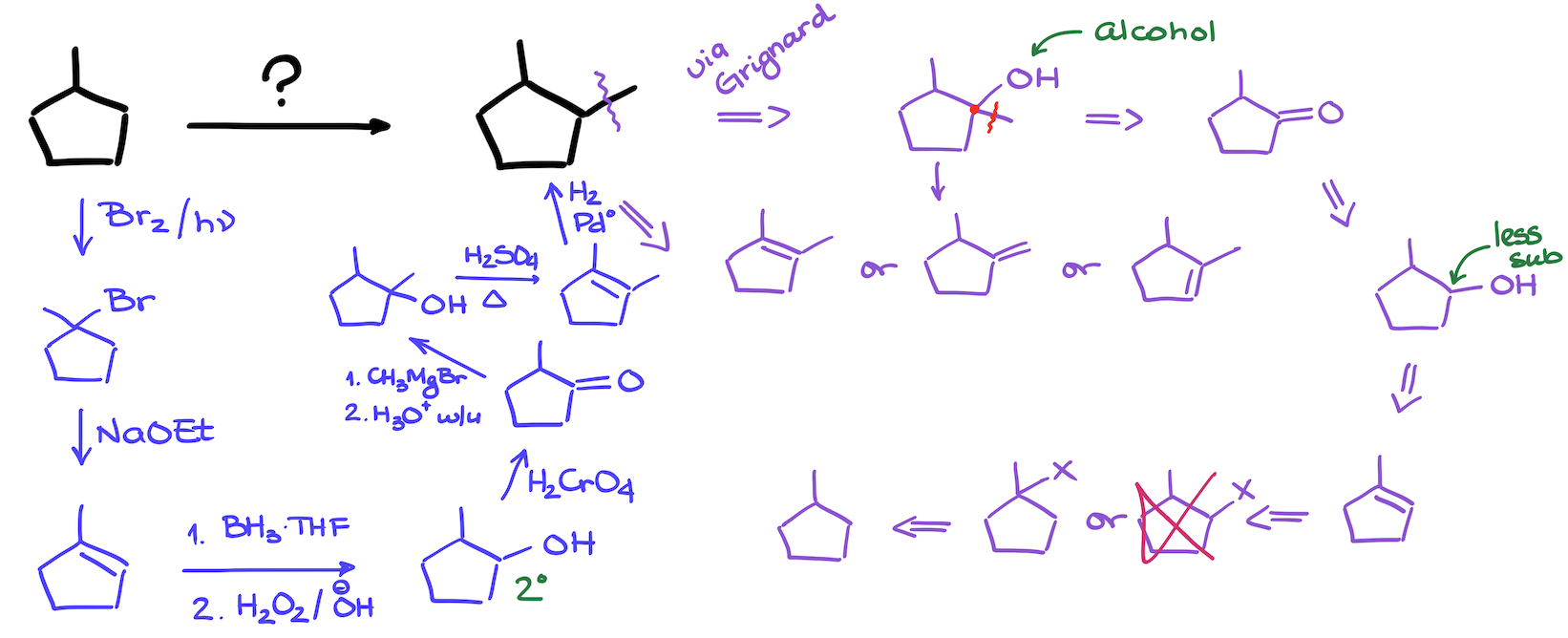
07. Synthesis of 1,2-Dimethylcyclopentane from Methylcyclopentane
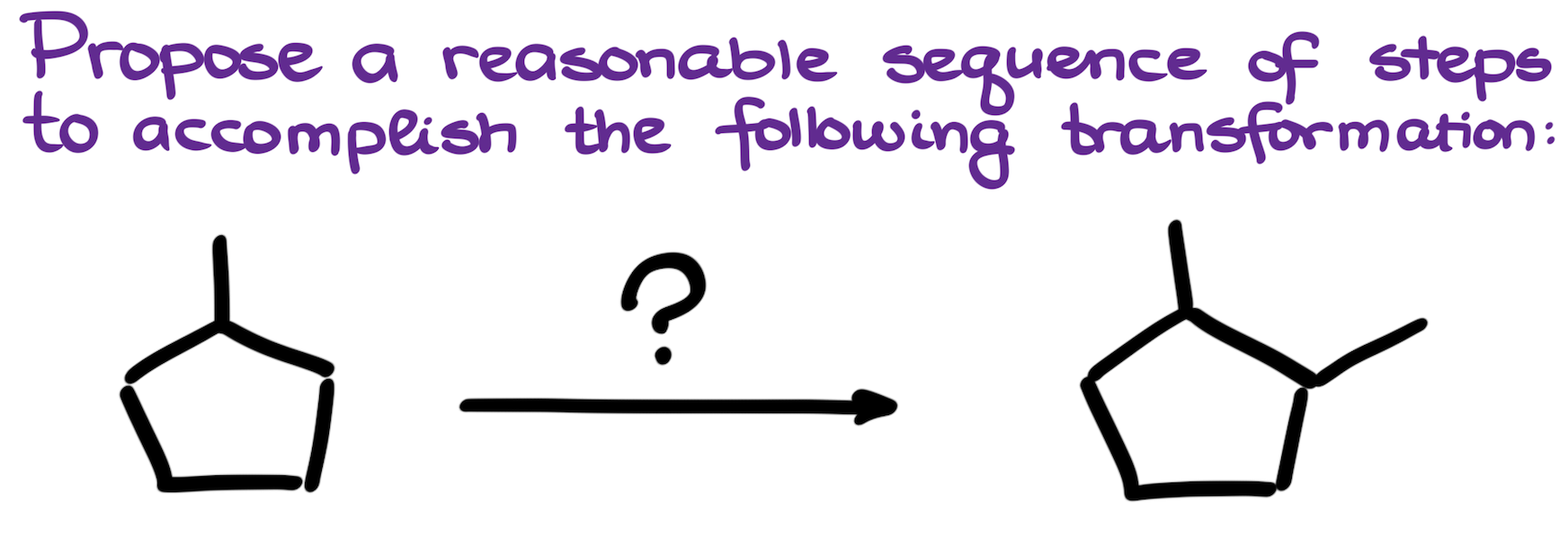
This is a classic style of a synthesis where we’re starting with a simple alkane and need to make a new carbon-carbon bond somewhere in the molecule without adding any other functional groups. Of course, you cannot just stick a carbon chain to a molecule that doesn’t have any functional groups. Which means, that we’re first going to add functional groups here, make our carbon-carbon bonds, and then remove all the leftover functional groups to get the final result.
Reactions that we see in this synthesis are:
- Radical halogenation
- Grignard reaction
- Elimination
- Hydroboration-Oxidation
- Oxidation of alcohols
- Dehydration of alcohols
- Reduction of alkenes