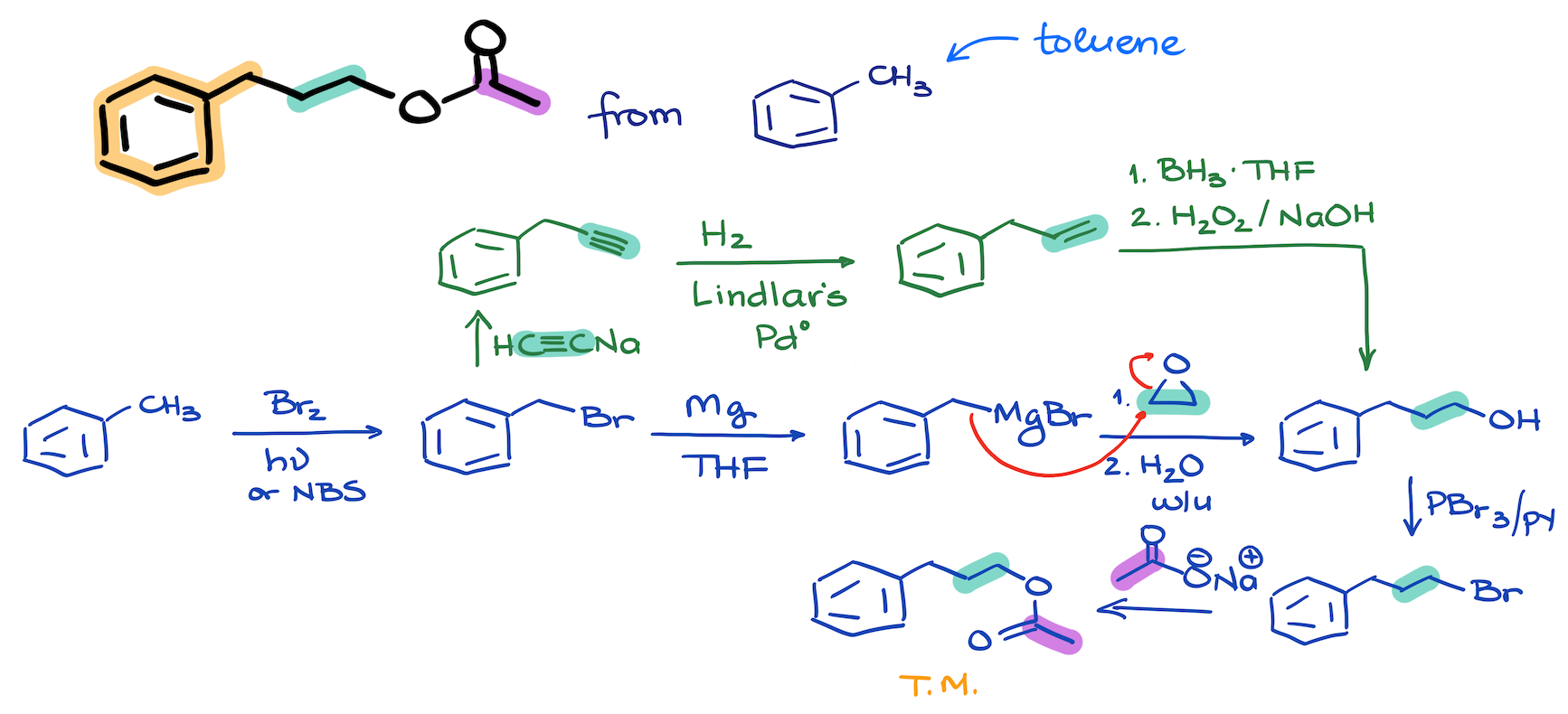
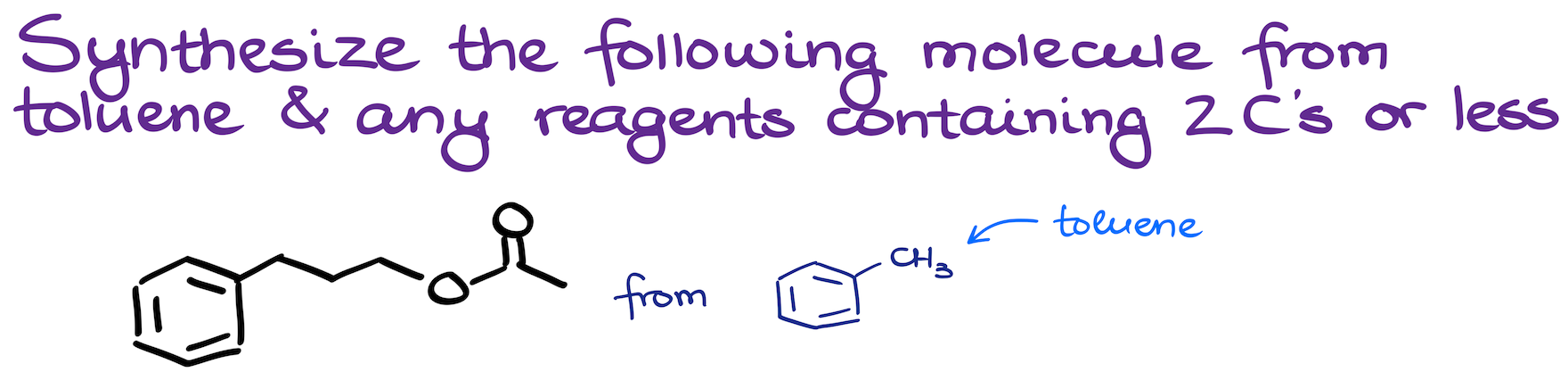
06. Synthesis of a Complex Ester from Toluene
The tricky part of this synthesis is to find where each piece of the puzzle maps onto the final products. Early in the organic chemistry curriculum, students know only one method of carbon-carbon bond-making, and that is the reactions of alkynide ions. And this synthesis certainly takes advantage of this reaction.
Another important point to keep in mind for syntheses like this one is that our limitation is the two carbon building blocks. We are not limited by the type of a functional group we can use. Which means, we can make those two carbon pieces as complex as we need for our purposes. So, in addition to doing the reaction of alkynide ions, we can do a Grignard reaction with an epoxide to make our carbon-carbon bond here.
Reactions that we’ve used in this synthesis are:
- Radica halogenation
- Grignard reaction
- Reactions of alkynide ions
- Conversion of an alcohol into alkyl halide
- SN2 substitution
- Hydroboration-Oxidation
- Partial reduction of alkynes