33. Aromatic Synthesis Challenge
In this tutorial, I want to talk about the synthesis shown here. We’re starting with a simple benzene molecule, and the goal is to end up with three different substituents on the aromatic ring.
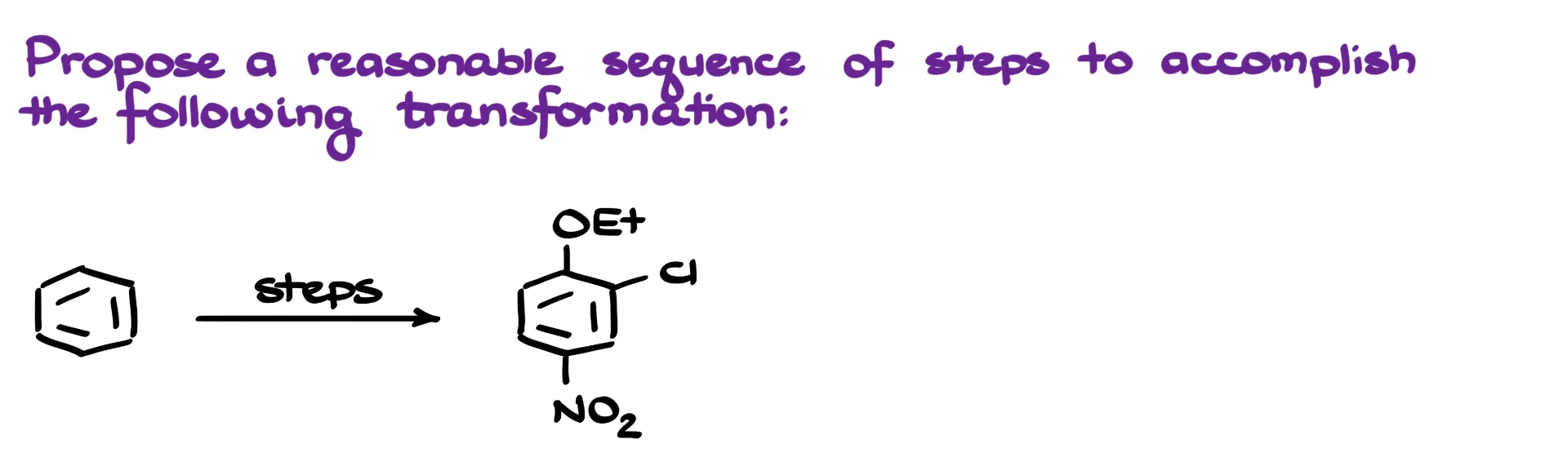
Looking at the substitutions, I notice a chlorine on the ring—that’s an easy one. We can introduce it using a standard halogenation reaction. The nitro group is also straightforward; we can add that via nitration. However, the ethoxy group at the top of the ring presents more of a challenge. There’s no direct method to add oxygen-containing groups like that through electrophilic aromatic substitution, so we’ll need to approach this part a bit differently.
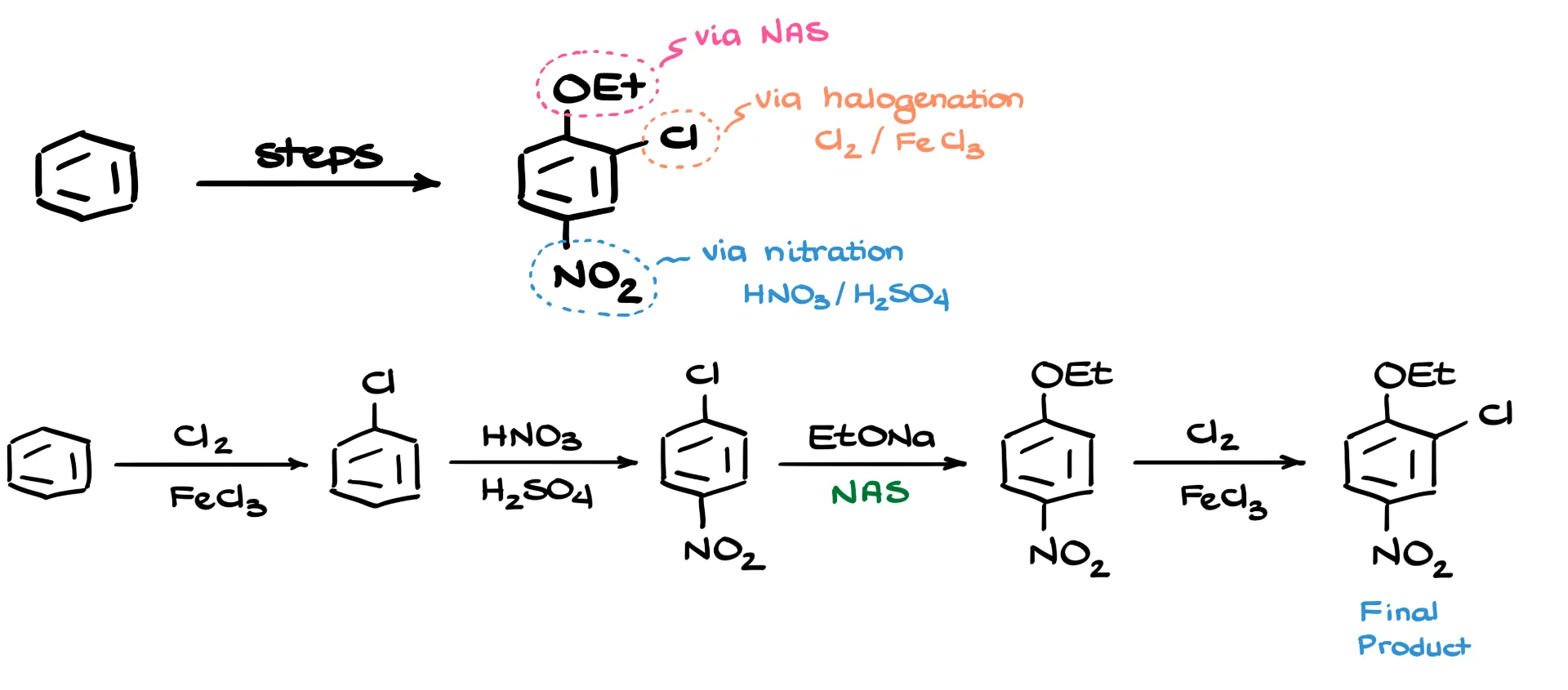
The most efficient route here is to use nucleophilic aromatic substitution. Fortunately, our target molecule contains an electron-withdrawing group—specifically, a nitro group—in the para position relative to where we want to add the ethoxy group. That makes nucleophilic aromatic substitution feasible at that position.
To build the synthesis, I’ll start by redrawing benzene as my base structure. The first step is a simple chlorination, which gives us chlorobenzene. Next, I perform nitration. Since chlorine is an ortho/para director, the nitro group ends up in the para position relative to the chlorine, giving me para-nitrochlorobenzene.
Now comes the key step: nucleophilic aromatic substitution. I react the para-nitrochlorobenzene with sodium ethoxide. The ethoxide replaces the chlorine, yielding the desired ethoxy-substituted nitrobenzene. Finally, to complete the synthesis, I add my last chlorine using another simple halogenation reaction, which gives me the final product.
So, what do you think of this synthesis? Let me know in the comments.
