34. How to Insert a Nitrogen Into a Ring
In this tutorial, I want to walk through a synthesis where we’re starting with methylcyclopentane and aiming to make a substituted piperidine as our final product.
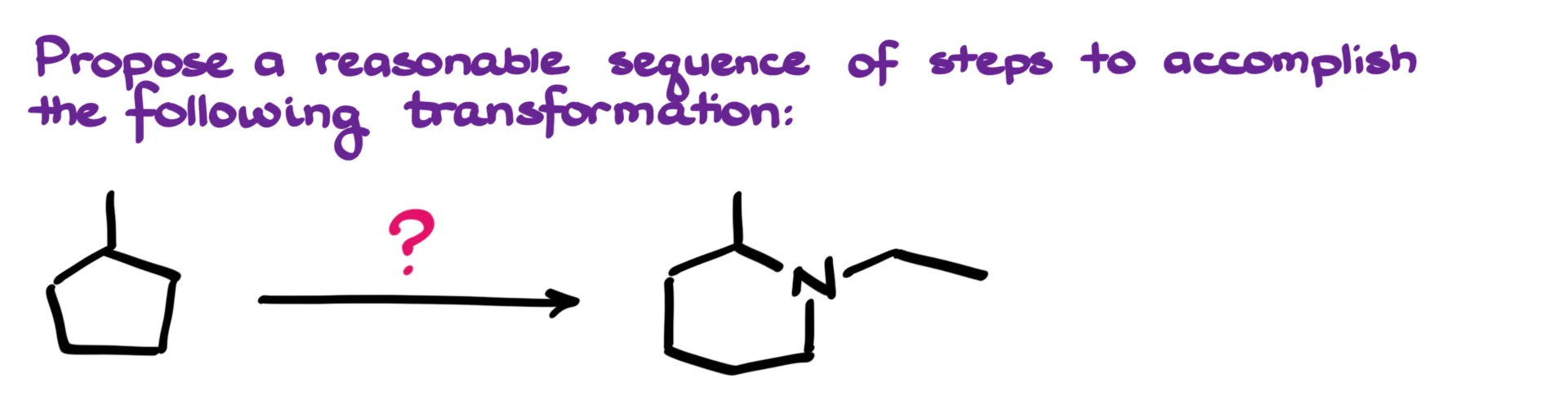
The first thing that jumps out right away is that we begin with a five-membered ring and end up with a six-membered ring. That tells me we’ll need to break the original ring open at some point and then reassemble it later during the synthesis. Functionally, we’re introducing an amine group, which isn’t anything too complex. So the overall plan becomes clear: break open the ring using ozonolysis and then form the amine via reductive amination.
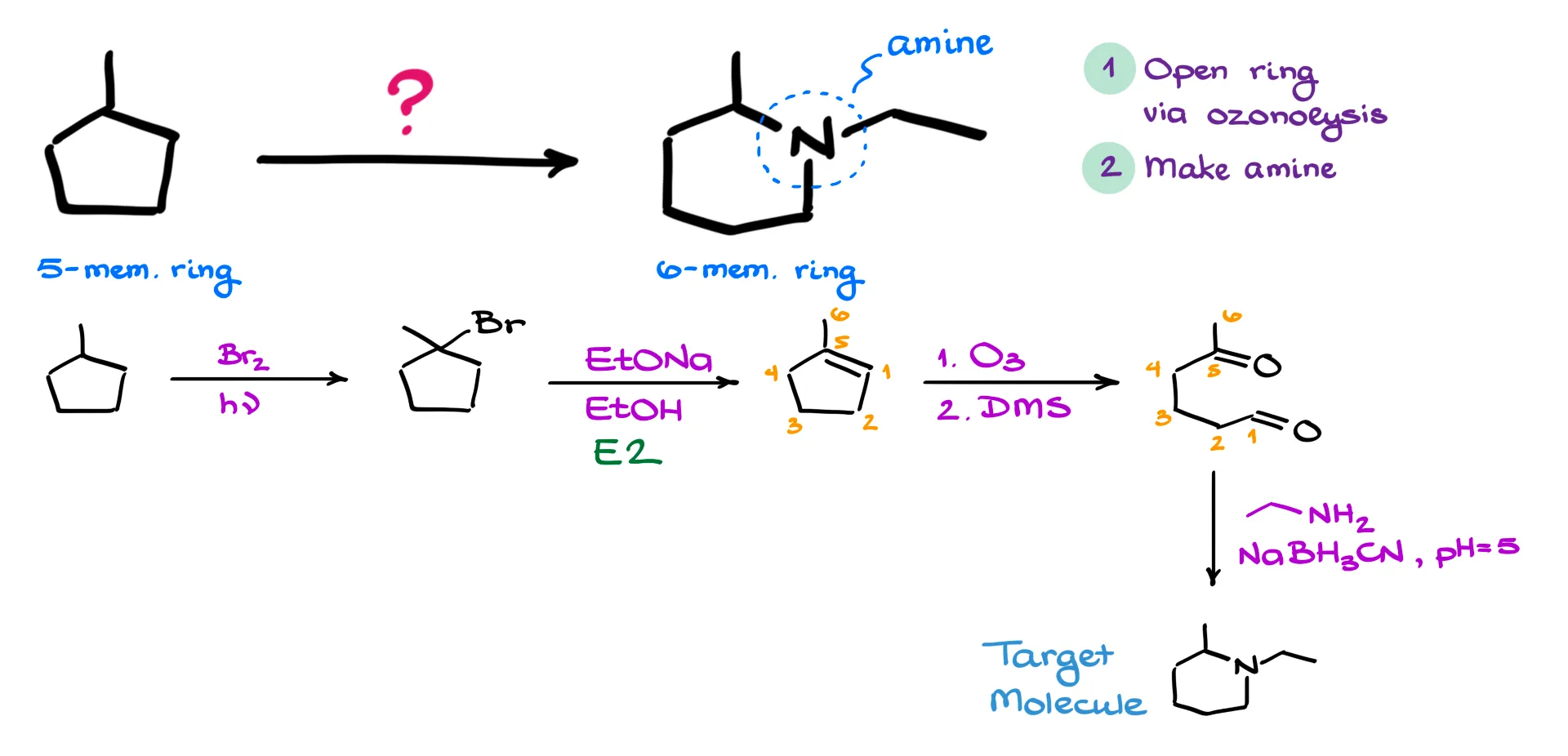
To start, I’ll redraw the starting material—methylcyclopentane. Since I need to do ozonolysis to open the ring, I first need to introduce a double bond. We don’t have any functional groups to work with initially, so the logical first step is a simple radical halogenation. That gives us a halogenated intermediate.
Next, I perform an E2-style elimination reaction to form the alkene. With the double bond now in place, I can carry out the ozonolysis to cleave the ring. At this stage, I like to double-check my carbon count to make sure I haven’t lost track of anything in the redrawing process.
The final step is to close the ring back up and simultaneously introduce the amine group. Since we’re forming two carbon–nitrogen bonds—not literally at the same time, but sequentially—the ideal method here is a one-pot reductive amination. This transformation allows us to form the ring and install the amine in a single synthetic operation. If you need a refresher on how reductive amination works, I’ve got a dedicated tutorial for that—check the description below for the link.
So, what did you think of this synthesis? Did you like the approach?
