35. Final Exam Style Synthesis
In this tutorial, we’re going to work through a synthesis starting from a simple aromatic compound and building up to a structure containing an acetal and a meta-substituted iodine. If you want to try solving this synthesis on your own first, pause the tutorial now—I’m about to dive into the preliminary analysis.

The first thing that stands out is the presence of an acetal functional group. That’s a fairly easy transformation—we can form it by reacting the corresponding ketone with a diol, typically in the presence of an acid catalyst. So this part of the molecule is something we’ll tackle toward the end of our synthesis.
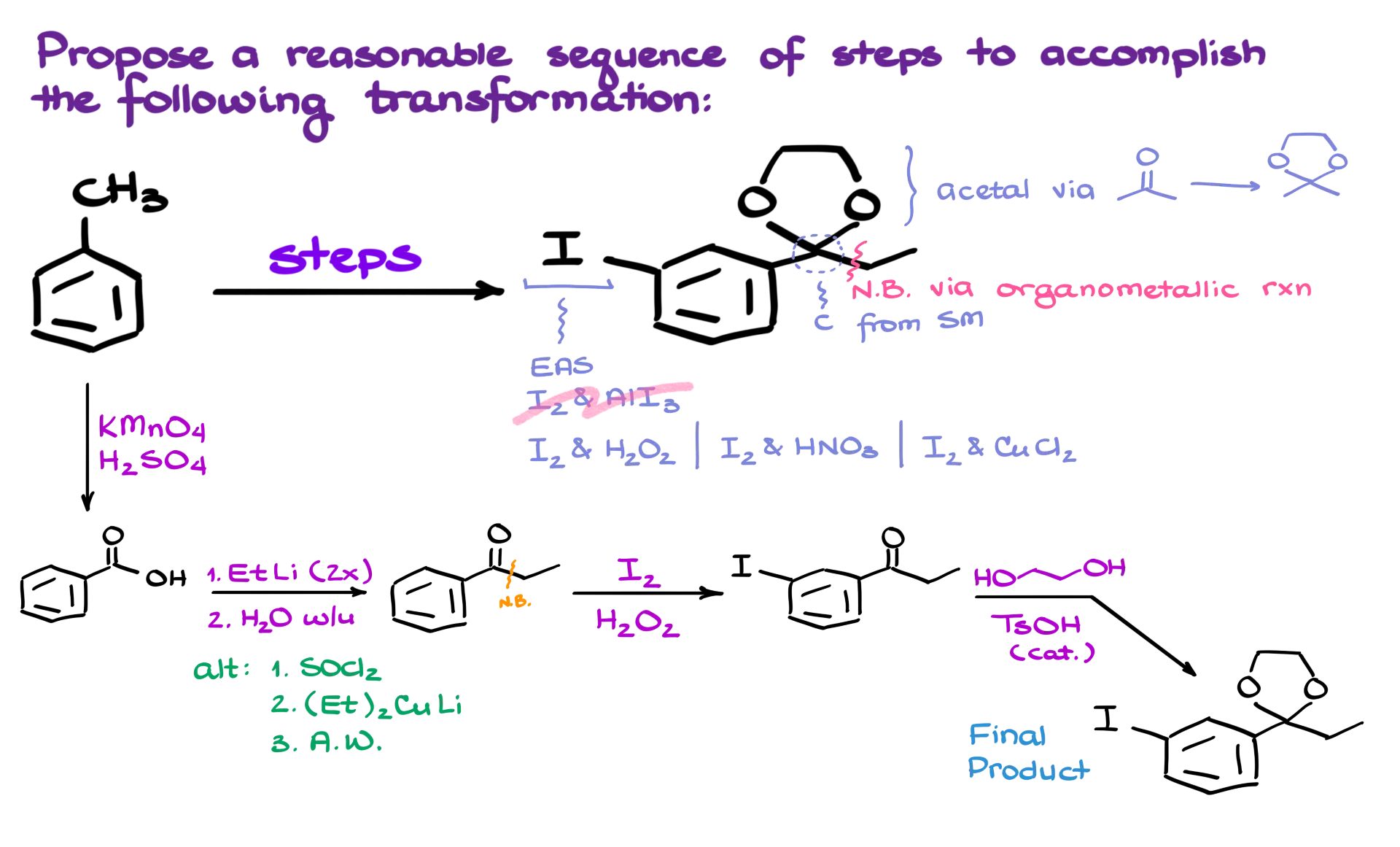
Next, I want to preserve this carbon from the starting material. It’s generally a good rule in synthesis planning to retain as many atoms as possible, avoiding unnecessary steps like chopping off carbons and re-adding them later. That kind of efficiency makes synthesis more straightforward. The carbon–carbon bond in the product is most likely the result of an organometallic reaction. We’ve got a few options here, and I’ll walk you through them as we go.
Now, the iodine in the meta position is an important feature. The simplest way to install that kind of group is through electrophilic aromatic substitution. But here’s the catch—while chlorination or bromination can be done using Lewis acids like AlCl₃ or FeBr₃, the same approach won’t work for iodine. Iodination requires more specialized reagents. There are several ways to do it, each with its own mechanism, but the bottom line is they’re all forms of electrophilic aromatic substitution and that’s the strategy we’ll use here.
Let’s jump into the synthesis. I’ll start with a benzylic oxidation to convert our starting methyl group into a carboxylic acid, giving us benzoic acid. From here, to form the new carbon–carbon bond, I’m going to treat this acid with ethyllithium. I’ll need two equivalents—one for deprotonating the acid in an acid–base reaction and the second to perform the nucleophilic addition.
This gives me a ketone as my intermediate. If you’re unfamiliar with how this transformation works, I’ve got a dedicated tutorial that walks through the process step by step—check the description for the link. Now, if I didn’t want to use organolithium chemistry, I could instead convert the carboxylic acid into an acid chloride and react it with a Gilman reagent (an organocuprate). That’s another solid route, and I also have a tutorial covering that method. Either way, this gets us to our ketone intermediate quickly and efficiently.
Once we have the ketone, we move on to installing the iodine. Since the aromatic ring is deactivated by the ketone, we need a more aggressive reagent to ensure successful iodination. I’ll use a reaction involving hydrogen peroxide for this step. This gives us the iodine in the meta position, right where we want it.
Finally, we perform our acetal formation. Treating the ketone with ethylene glycol and p-toluenesulfonic acid as a catalyst completes the transformation. This yields our final product—an aromatic compound with an acetal and a meta-positioned iodine.
So, what did you think of this synthesis? Was it an interesting challenge, or did it come together more easily than expected? Let me know what you thought!
