36. Another Final Exam Synthesis
In this tutorial, I want to walk through a transformation where we start with a plain benzene molecule and end up with a substituted aromatic amide. If you want to try solving this synthesis on your own first, go ahead and pause the tutorial now—I’m about to dive into my preliminary analysis.
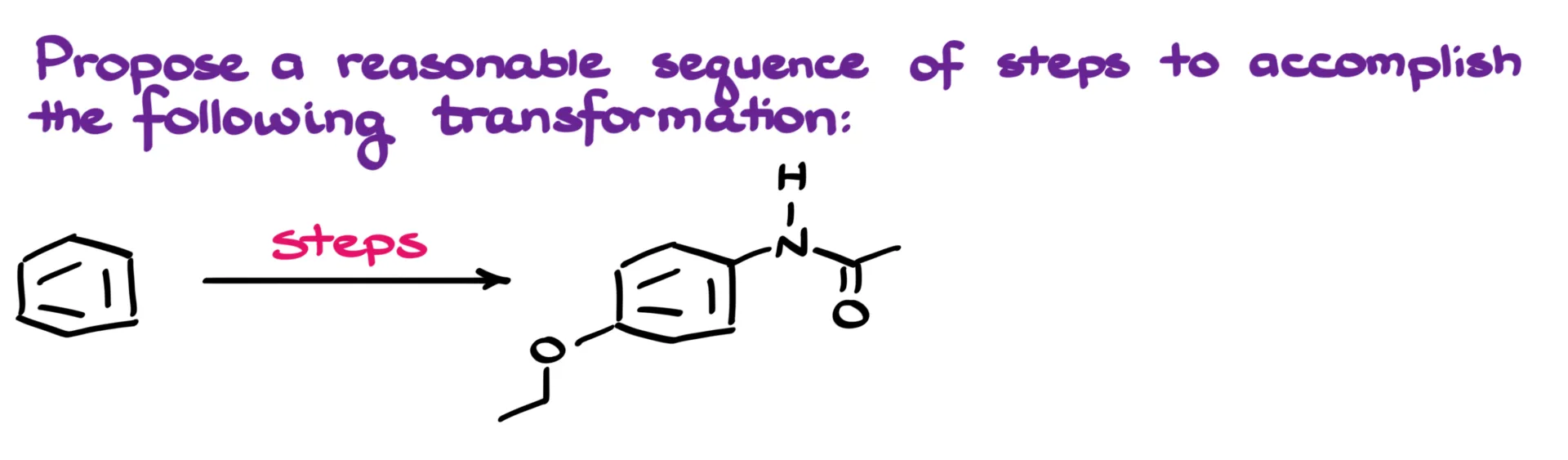
The first thing that stands out to me is the amide functional group. This one’s pretty straightforward. We can assemble it easily through a simple acyl substitution reaction, so there’s not much to worry about here.
The more interesting challenge lies in the nitrogen attached to the aromatic ring. Ideally, that would be an amine, but we can’t directly install an –NH₂ group on a benzene ring through electrophilic substitution. So instead, we’ll introduce a nitro group first—since that’s easy to install—and then reduce it to an amine in a later step.
Then there’s the ether group. While there are a few methods for forming aryl ethers, the most convenient in this case is going to be a nucleophilic aromatic substitution. For that, we’ll need an electron-withdrawing group on the ring to make the substitution possible.
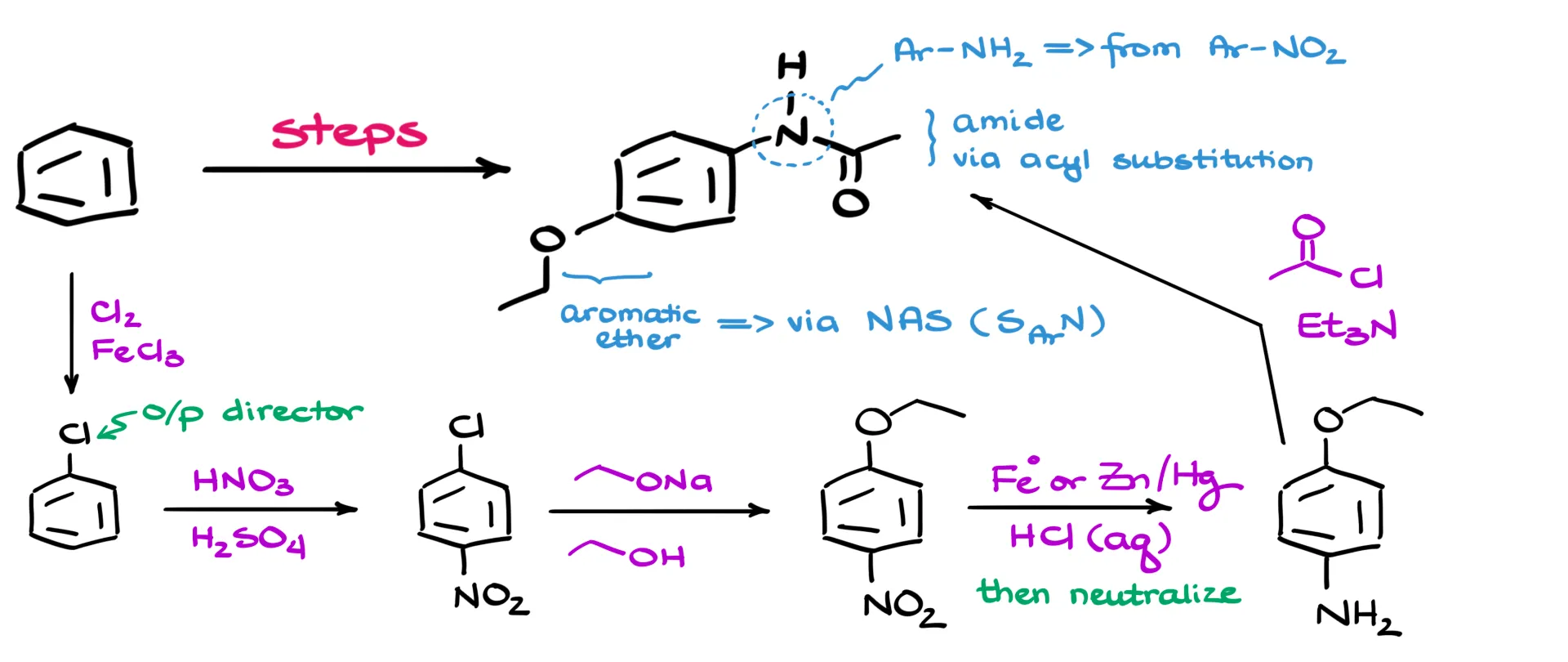
With that game plan in mind, here’s how the synthesis will go. We start with a simple halogenation of benzene to produce chlorobenzene.
Since chlorine is an ortho/para-director, we can then perform nitration. This places the nitro group in the para position relative to the chlorine—perfect for our purposes. Now, with a nitro group on the ring, we’re all set up to do a nucleophilic aromatic substitution. We react the compound with ethoxide, replacing the chlorine with an ethoxy group and giving us para-ethoxynitrobenzene.
At this stage, the nitro group has served its purpose, so we go ahead and reduce it. There are tons of ways to do this—you can use iron and acid, a Clemmensen-style reduction, complex hydrides, or hydrogen gas with a heterogeneous catalyst if you’ve got the setup. The specific method doesn’t matter; what matters is that we successfully reduce the nitro group to an amine.
Finally, the last step is a straightforward acyl substitution to form the amide, giving us our final product. EZPZ!
Let me know what you thought of this synthesis and if you would’ve approached it differently.
