38. Synthesis of a Complex Bicyclic Vinyl Halide
In this tutorial, I want to go over an interesting synthesis where we start with a simple benzene molecule and end up with a bicyclic vinyl halide.
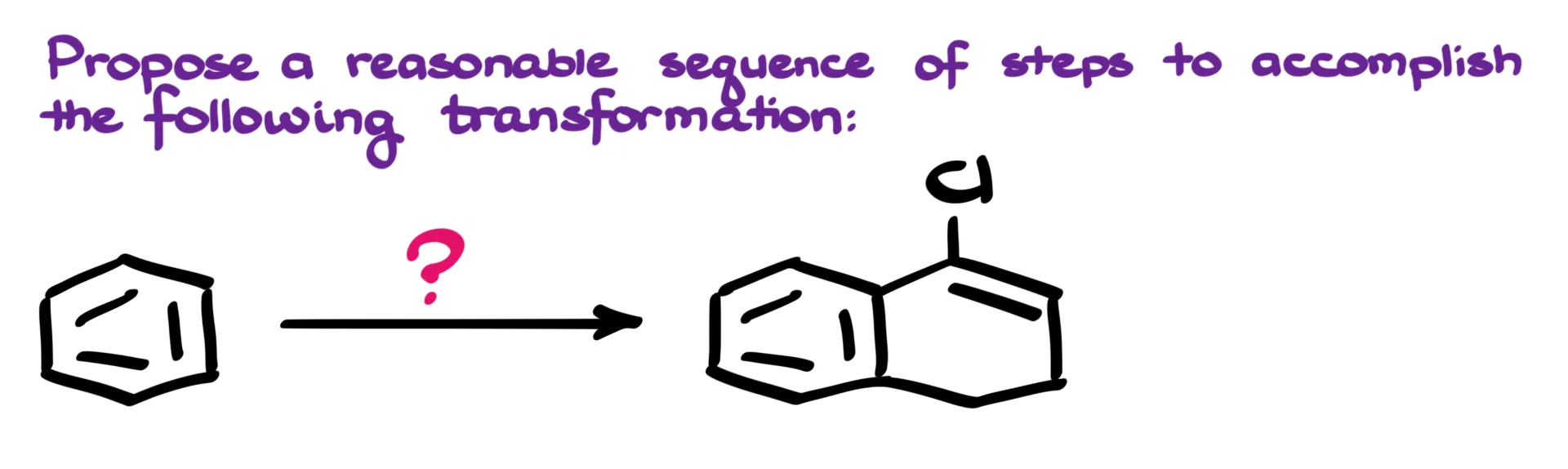
Right away, it’s clear that we need to add a large portion to the benzene ring. That means we’ll be forming two new carbon–carbon bonds in this process. While there are several possible approaches for assembling this kind of bicyclic skeleton, the most straightforward route is the classic α-tetralone synthesis.
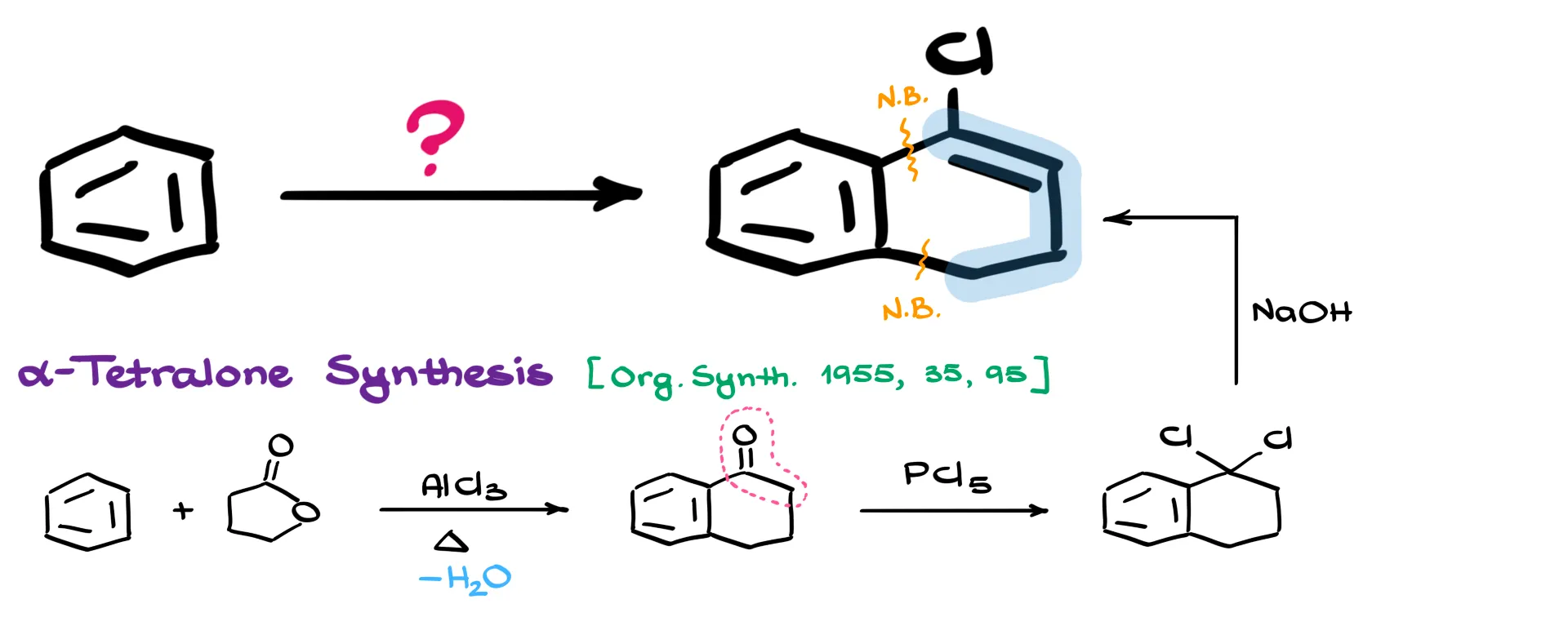
The α-tetralone synthesis is a well-established procedure where benzene reacts with γ-butyrolactone in the presence of a Lewis acid catalyst—like aluminum chloride—to produce tetralone in a single step. It’s a clean, efficient way to introduce the bicyclic framework we need.
Once we have tetralone, the next task is to convert the carbonyl group into a vinyl halide. That means we’ll need to replace the oxygen with chlorine and form a double bond. For that transformation, I’m going to use a reaction that’s not typically covered in introductory organic chemistry, but it’s incredibly useful to have in your synthetic toolkit.
If we treat ketones with phosphorus pentachloride (PCl₅), we get the corresponding geminal dihalide. This reaction isn’t the most pleasant one to carry out—working with PCl₅ can be a bit rough—but it generally gives good yields and eliminates the need for multiple separate steps.
With the geminal dihalide in hand, we perform a simple elimination reaction. In this case, I’m using sodium hydroxide (NaOH) as the base. That gives us the desired double bond and completes our synthesis.
What did you think of this one? Would you have been able to figure it out without knowing about the less commonly taught reactions we used here? Let me know your thoughts in the comments.
