Oxidation of Alcohols: Overview
Alcohol oxidation is something that you are already intimately familiar with because right now your body, namely your liver, is oxidizing the alcohols that you have in your bloodstream that you get from your diet into corresponding aldehydes, ketones, and carboxylic acids.

And you might be like, “wait a minute, I don’t drink, so I don’t have any alcohol in my blood!” Not so fast! Not all alcohols that we consume is ethanol, that you’re probably thinking about right now.
Ever heard of xylitol or sorbitol? Those are alcohols. Yeah, those sweet-sweet lies with “no sugar added” you read about on the label. And what does your body do to the alcohols like sorbitol? Yep, it oxidizes them into corresponding carbonyls like fructose and glucose. So much for “no sugar added” sigh but I digress.
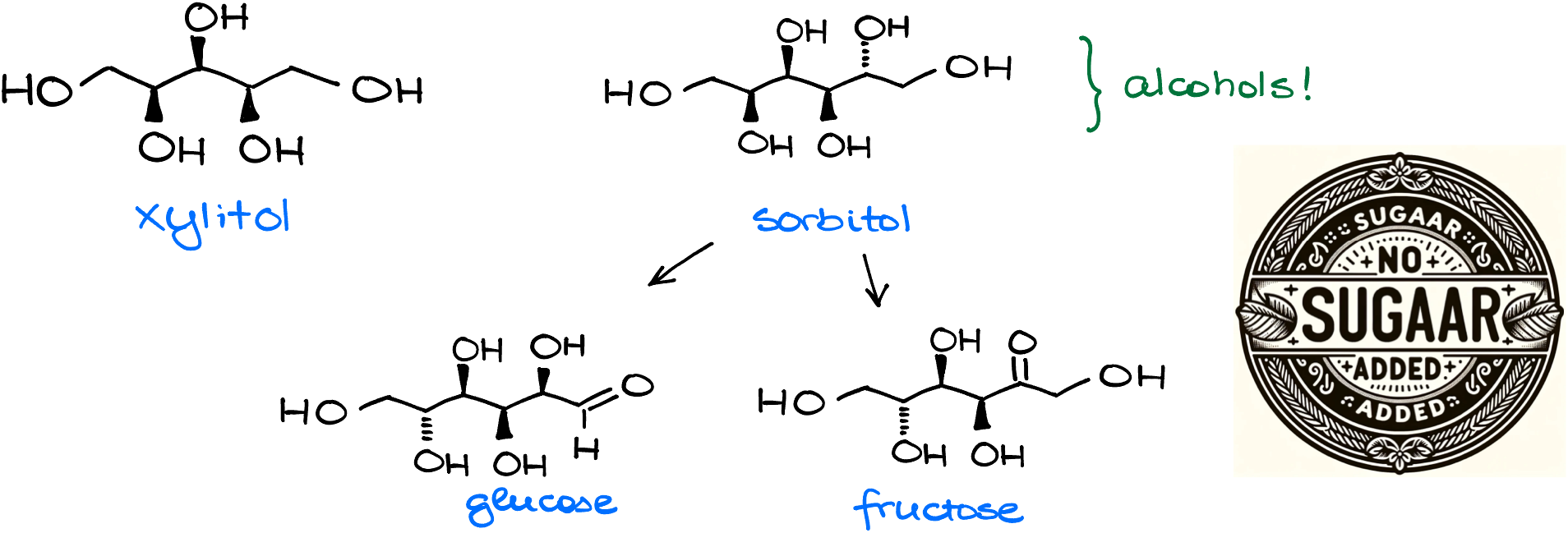
In the body, we use alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ADLH) enzymes to deal with the alcohol oxidation. If the first one works slow, you’re known as a cheap date, if it works too fast, you’ll be hugging the porcelain throne after a single shot coz acetaldehyde is pretty toxic to us, but if both are working extremely efficiently, you’ll outdrink the entire Irish pub and then some.
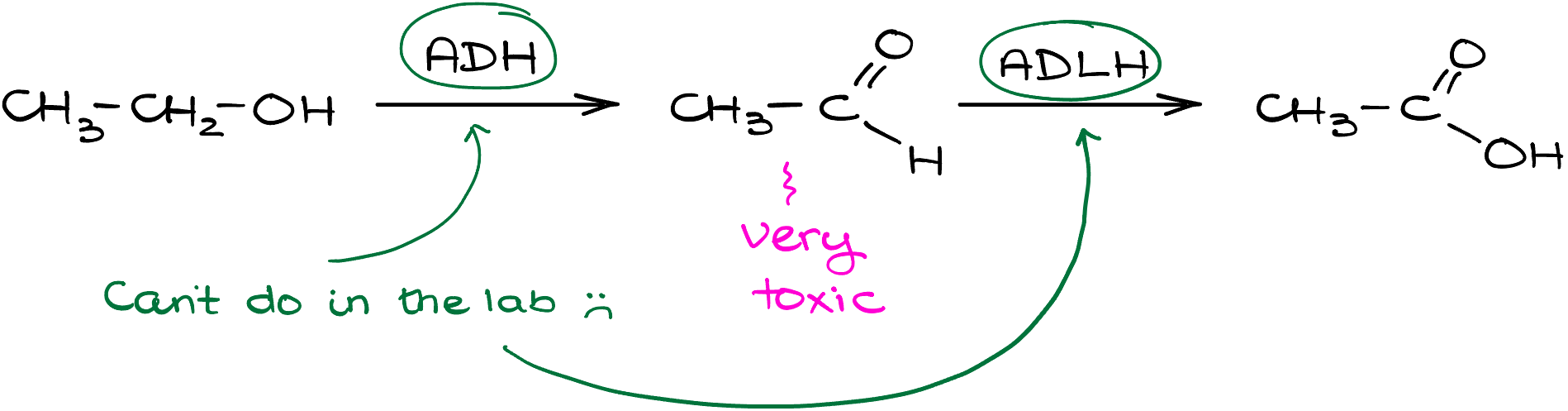
In the lab however, we do not have the luxury of the mobile biofactory we call liver stuffed with various enzymes to help us with the oxidation reactions. So, we need to use other techniques.
Overview of the Oxidation Process in General
But before we dive into the reactions themselves, I wanna make sure you have a firm grasp on what the oxidation means to begin with.
Oxidation refers to the formal loss of the electrons. Since we’re dealing with the covalent compounds in organic chemistry, we’re not going to see a complete transfer of electrons from one element to another one. Rather, we’re going to see the formation of new chemical bonds that will make atoms more or less electron rich.
Let me illustrate it with an example.
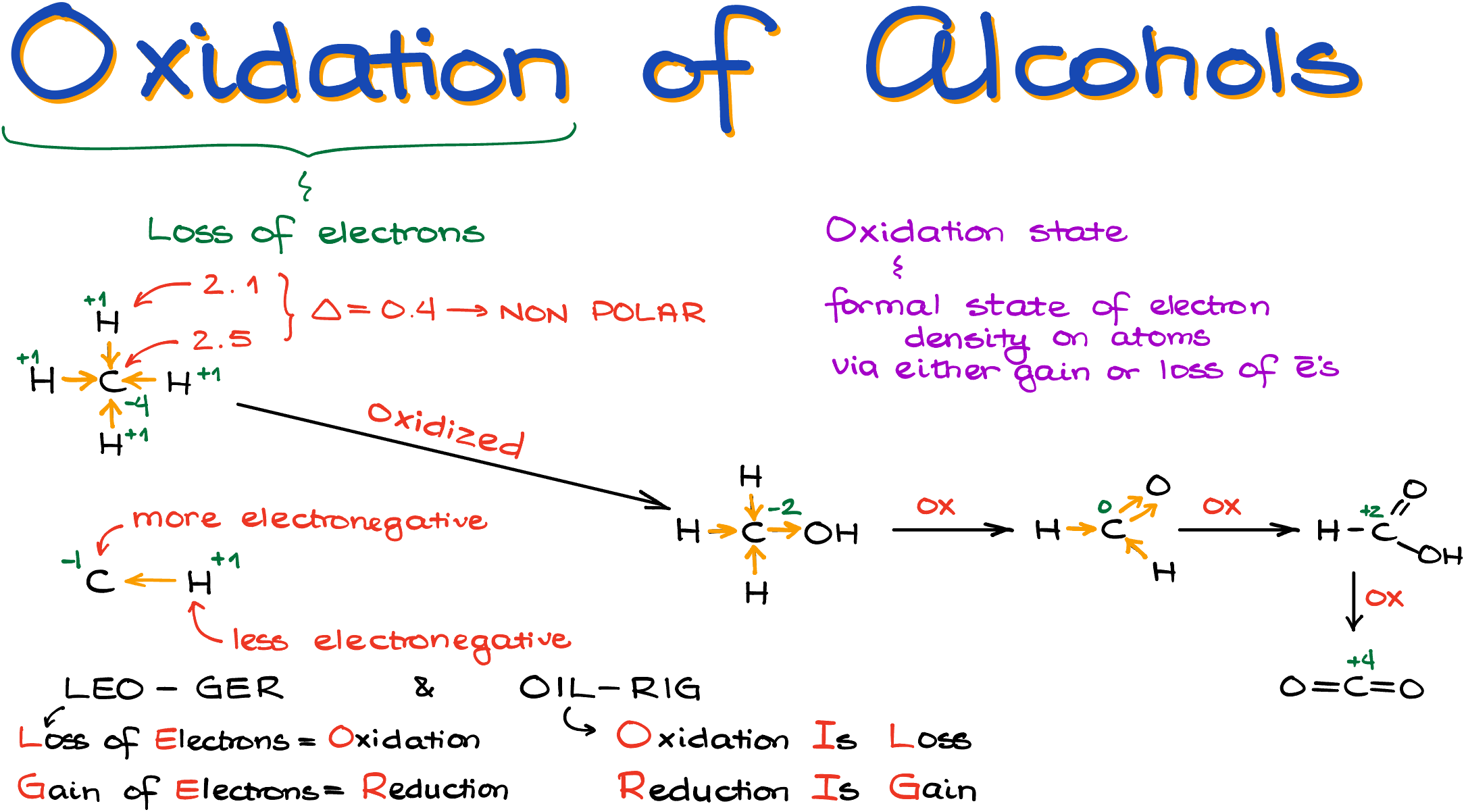
Say, we have a methane molecule. The electronegativity of carbon is about 2.5, while electronegativity of hydrogen is about 2.1. From what we remember from general chemistry, we know that that makes a non-polar bond since the difference in the electronegativity is rather insignificant. This also means that neither carbon, nor any of the hydrogens will have an excess or a lack of electron density. Electrons are shared more or less equally.
Bond polarities, however, doesn’t matter much for the red-ox reaction. When we’re talking about the oxidation of molecules, we’ll often be dealing with the term “oxidation state” that describes the formal (or not so formal) electron deficiency or electron excess of our atoms. The oxidation states are assigned based on the differences in the electronegativities of atoms making up the bond.
And, when it comes to the oxidation states, they don’t care how big or small those differences are. If there’s a difference in electronegativity, then we’ll formally assign higher or lower oxidation states to atoms. The only time when the bond to an atom doesn’t influence the oxidation state, is when an atom is connected to itself.
So, here, I have a carbon with a slightly higher electronegativity, and hydrogens that have slightly lower electronegativity. Although, the difference is quite small, it is there. And that’s the only thing we care about for the purposes of the oxidation states. Thus, we’ll say that each bond is polarized—and I’ll emphasize it one more time—formally, towards the carbon atom. This means that my carbon will have the oxidation state of -4 and each hydrogen will have the oxidation state of +1. Importantly, those are not the charges on the atoms! You can think about it as if the atom with the lower electronegativity pushes the electrons towards the atom with the higher electronegativity, or the atom with higher electronegativity is pulling electrons away from the atoms with the lower electronegativity. Either way work for our purposes.
So, the atom that pull electrons towards itself gets a -1 for each bond. And likewise, the atom that gives electrons gets +1 for each bond.
This way, if instead of one of those hydrogens in methane I have an -OH group, making my molecule into an alcohol, I now have a very polar bond between my oxygen and my carbon. This means that now, the carbon atom gets electrons from 3 bonds with hydrogens and loses electrons through one bond to the oxygen. Thus, it’s oxidation state is now going to be -2. It “gained” -3 from hydrogens and it “lost” +1 to an oxygen atom. And I know that “gaining” negative and “losing” positive feels counterintuitive, but remember–we’re talking about the gain and loss of the electrons here. And since electrons are the bearers of a negative charge, we’re “gaining” a negative with them.
So, since the carbon formally lost the electrons because it went from the oxidation state of -4 to the oxidation state of -2, it has been oxidized.
This is also where the common mnemonics LEO-GER or OIL-RIG comes from. LEO = losing electrons is oxidation; GER = gaining electrons is reduction; OIL = oxidation is loss; RIG = reduction is gain.
If my alcohol, methanol here, is oxidized again to an aldehyde, formaldehyde in this case, we’re going to have a carbon with the oxidation state of 0 now. And yes, atoms can have an oxidation state of zero even if they are not in the elemental from.
I get zero here because my carbon “gains” electrons from two bonds to the hydrogen atoms, and it “loses” electrons through two bonds to the oxygen. Notice that we are counting the double bond as, well, a double bond for these purposes.
This way, if we continue with this lineup, we’ll end up with the formic acid, where the carbon’s oxidation state is +2. And finally, carbon dioxide with carbon at the oxidation state of +4.
This is the example for the simplest alcohol. How would it look like if we make it a little more complicated and look at the methanol’s brother—ethanol?

We’ll get a similar lineup where we start from ethanol, move to acetaldehyde, and then finish this lineup with acetic acid. The oxidations states here are going to be -1, +1, and +3 for the carbon that keeps getting bonds to the oxygen. The carbo-carbon bond makes no difference to our oxidation states, so it’s neither pulling nor pushing electrons.
As you have already noticed, when we’re talking about the oxidation of alcohols, we’re only focusing on the oxidation of carbons. This is going to be a common trend for all red-ox reactions in organic chemistry—we only care about the carbon-containing molecules and ignore everything else. As the matter of fact, we’ll pretty much ignore everything but the carbons for our red-ox reactions!
Now, when you have a better idea of how oxidation of alcohols work and how we determine the oxidations states, let’s actually look at the reactions that make this all happen. Within the scope of this tutorial, I’m not going to be talking about the mechanisms of the reactions themselves. Here, I want you to build the general understanding of what’s going on with the transformation. This way you’ll be able to analyze your substrates, predict if the reactions is possible, and predict the products themselves. I see a lot of students focus on the mechanisms and lose the forest for the trees. If you want to learn the details of the mechanisms for each reaction in this video, I’ll have dedicated tutorials for those.
How Structure of the Alcohol Determines the Outcome of the Oxidation
The oxidation of alcohols produces different products depending on the nature of the starting material—the alcohol—that we are going to use in our reaction. We classify alcohols as primary (1°), secondary (2°), and tertiary (3°). This classification is based on how many other carbons are connected to the carbon with the -OH group.
So, for instance, here I have a primary alcohol (butane-1-ol), the secondary alcohol (butane-2-ol, or sec-butanol), and a tertiary alcohol (2-methylpropan-2-ol, or tert-butanol).
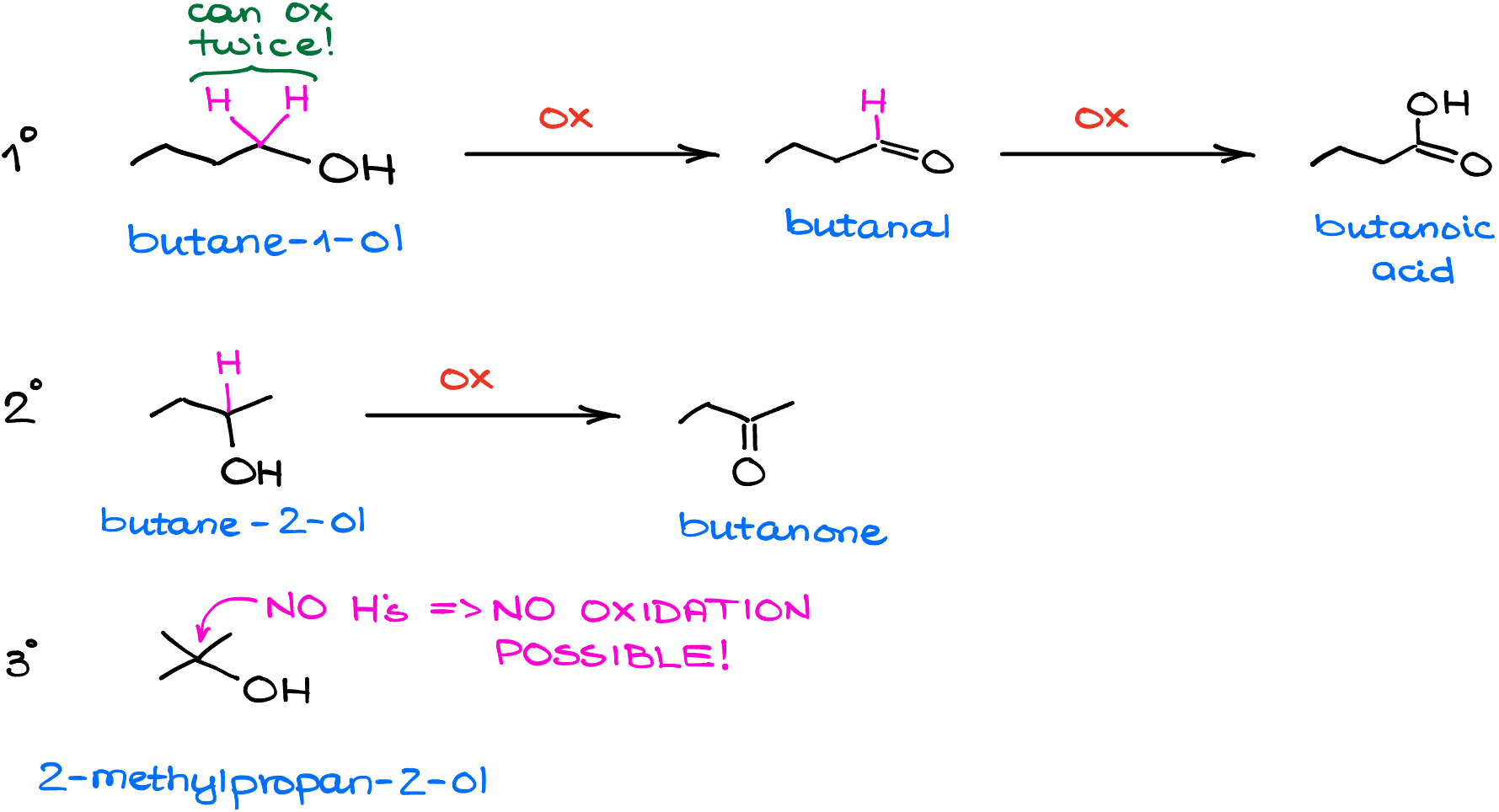
The main requirement for the oxidation reaction is the presence of a hydrogen on the carbon with the -OH. Thus, primary and secondary alcohols can be oxidized, while the tertiary alcohol will stay unchanged no matter how many shamanic dances you’re going to perform around it.
Also, since the primary alcohol has two hydrogens on the carbon with the -OH group, it will be able to undergo oxidation twice.
So, after the first round of oxidation, a primary alcohol makes an aldehyde. Butanal in this case. If we continue with our oxidation, it makes a butanoic acid. In the case of the secondary alcohol, it can only go through the oxidation once giving us the corresponding ketone. Butanone in this case.
And since primary alcohols can go through a two-stage oxidation process, you have to be mindful about the reagent choice. Some reagents will stop after the first round. Some, just like the express train, will go through the aldehyde stage without stopping there.
So, which reagent does what?
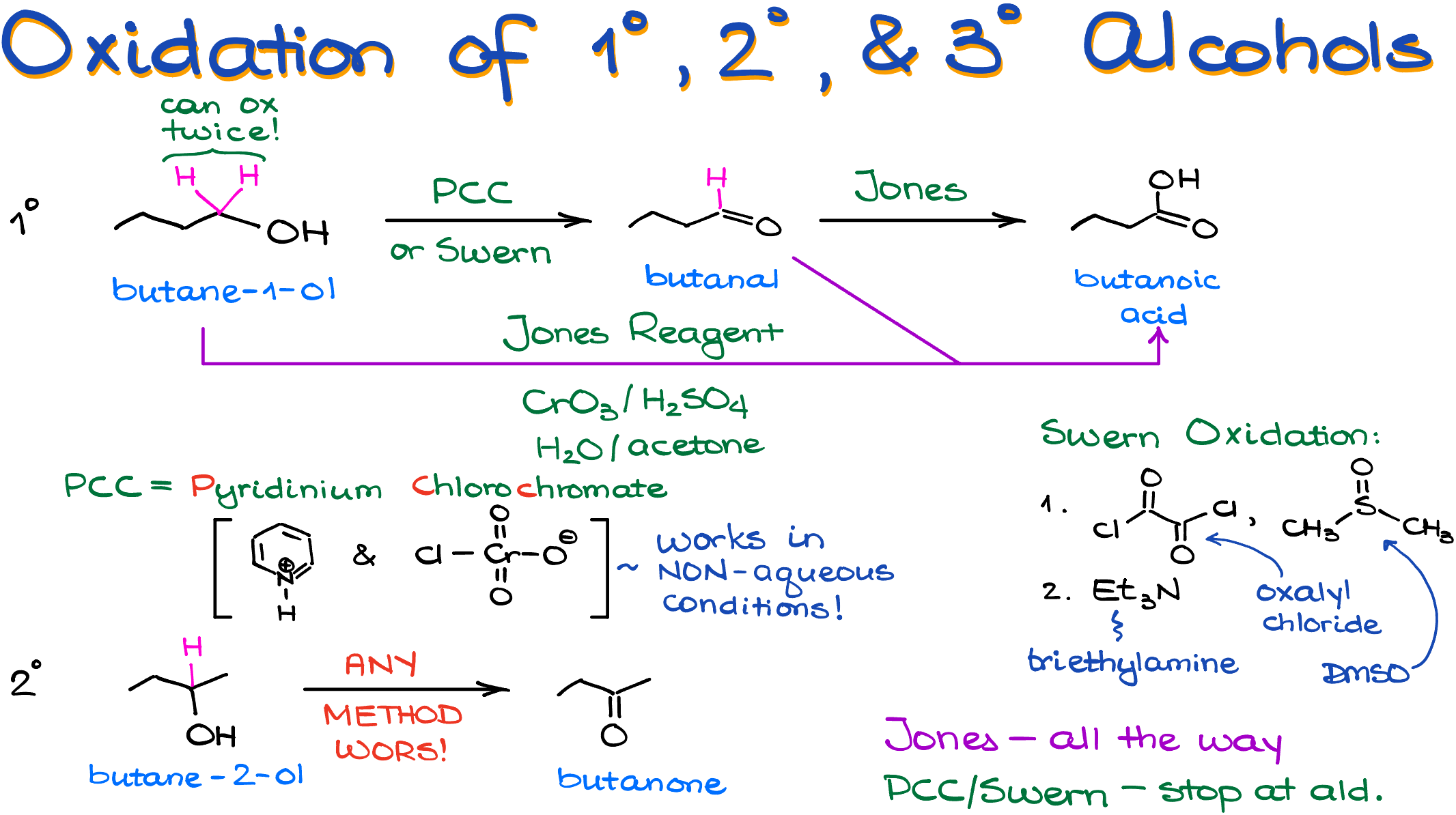
The most common “express train” you’ll see in your course is the Jones reagent. The Jones reagent is the mixture of chromium trioxide (CrO3) and dilute aqueous sulfuric acid (H2SO4) in acetone as a co-solvent. The reagent and the reaction are named after the discoverer of the method, Sir Ewart Jones, a British chemist, who first documented this method back in the 1940s. This technique laid the groundwork for synthesizing a variety of compounds, significantly impacting fields like medicine, biochemistry, and materials science. The problem with the Jones Reagent though is the toxicity of chromium. You see, while the Jones Oxidation is efficient and has historical significance, the chromium trioxide is a highly toxic and potentially carcinogenic substance. Prolonged exposure to chromium compounds can lead to skin irritation, respiratory issues, and even increase the risk of cancer. That’s why many modern labs are switching to safer, “green” alternatives for alcohol oxidation. However, since this is such a classic reaction, we still teach it to our students as the main go-to method for a complete oxidation of alcohols to carboxylic acids.
I also wanna point out that if you have an aldehyde as your starting material or an aldehyde functional group is present in your molecule, you will oxidize it along with any alcohols. While the Jones Oxidation might be one of those express trains that doesn’t stop at the aldehyde station, it’ll happily grab you and drag you along to the carboxylic acid final stop. So, keep that in mind when working on your oxidation reactions and don’t let your instructor trick you!
If we do want to stop at the aldehyde and not proceed to the carboxylic acid, we’ll use PCC, which stands for pyridinium chlorochromate. Complex with pyridine allows this reagent to easily dissolve in organic solvents, such as dichloromethane (DCM), allowing us to perform this reaction in non-aqueous conditions. The absence of water stops this reactions at the formation of the aldehyde and because of this, the oxidation with PCC is often termed as a “weak” or “mild” oxidation technique.
I’m going to go on a 5 second rant here, because the oxidizing agent in both PCC and the Jones oxidation is chromium and the outcome has nothing to do with the “strength” of our reagent. It’s the water that makes the difference. So, the PCC reagent that can be used in a non-aqueous conditions, stops at the aldehyde, while the Jones reagent that is used in an acetone-water solvent system, keeps reacting.
Also, PCC suffers from the same problem as the Jones reagent—chromium toxicity. PCC was first described by Elias J. Corey—a name you might recognize for his Nobel Prize in Chemistry—and his student William Suggs in 1975. At the time, the discovery (which was accidental) was revolutionary. However, nowadays, you’ll be hard pressed to find anyone actually using the PCC in the lab.
So, what are the alternative? Oh, a sea and a small lake! But the most common one and the one you’re likely to see in your class is the Swern oxidation.
The Swern Oxidation was developed by Daniel Swern in the 1970s when he was at the U.S. Department of Agriculture. Yes, you heard right—this non-toxic oxidation method came out of agricultural research! Not all chemical inventions happen at a university lab. Quite a few brilliant discoveries have originated from the industrial and other non-academic establishments. And while this reaction has been discovered at around the same time as the PCC, it never caught up till comparatively recently. I suspect it has something to do with the Elias Cory’s reputation and his overall influence on the field of organic chemistry, but I’m not going to speculate about it here.
Anyways, the Swern oxidation employs a mixture of oxalyl chloride and dimethyl sulfoxide (DMSO) as its reagents making a sulfur-containing intermediate, which then undergoes the intramolecular red-ox reaction promoted by non-nucleophilic bases like trimethylamine (Et3N). And while this is a mighty stinky reaction since it produces dimethylsulfide as a co-product, and I’ll tell you, I’ve done this reaction multiple times in my career and this guy stinks—this is a clean and reliable way to convert your alcohols to aldehydes without over-oxidizing them to carboxylic acids.
So, remember, if you need to take your primary alcohol all the way to the carboxylic acid, use the Jones Oxidation. If you wanna stop at the formation of the aldehyde, use either PCC or Swern.
What about the secondary alcohols? Well, since the secondary alcohols can’t be oxidized past the formation of the ketones, it really doesn’t matter what you use as your oxidizing agent. So, use whatever you love (or hate) the most.
Examples of the Oxidation of Alcohols
Are we ready for a few examples?
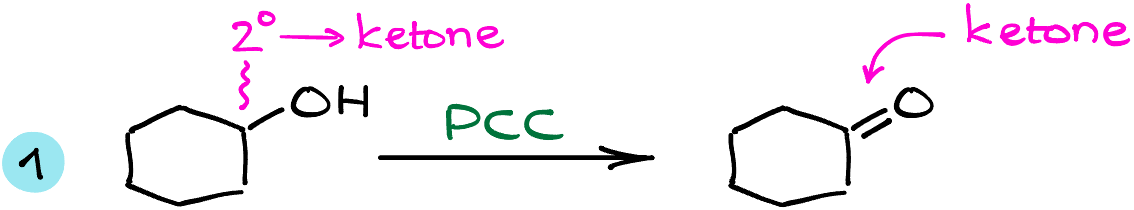
The first example here is a reaction of cyclohexanol with PCC. This is a secondary alcohol, so from what we have learned a few moments ago, we know that it will be oxidized to the corresponding ketone. To come up with the structure of my product, I’ll redraw the starting material, get rid of the hydrogen on the oxygen, and make a double bond between the carbon and the oxygen. Remember, that if you’re drawing the complete Lewis structure for this molecule, the hydrogen you had on the carbon with the -OH is also gone!
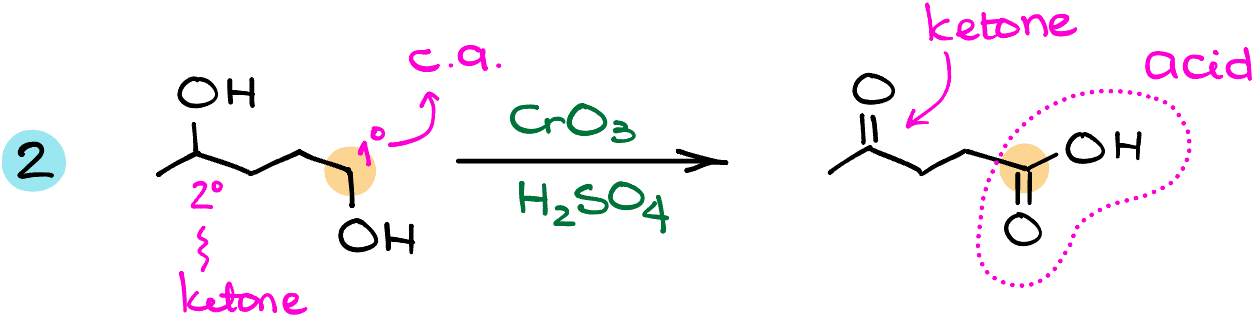
In the next example, I have the starting material with two alcohols in its structure. The common oxidation reactions typically oxidize all they can reach. So, here we’ll end up oxidizing both of our alcohols. The primary alcohol will be turned into the carboxylic acid, while the secondary alcohol will end up as a ketone.
Pay close attention to how you draw your molecules for these types of oxidations! The carbon with the -OH group becomes the carbon of the carboxylic acid. Don’t accidentally add an extra carbon to your molecule. It might seem like a “duh” moment, but this is an extremely common mistake I see student make all the time!

Ok, moving on, let’s look at this example. Here, I again have two -OH groups. One is the tertiary alcohol, while the other one is the secondary alcohol. We know that tertiary alcohols cannot be oxidized, so we’ll keep that one as is. The secondary alcohol, however, turns into a corresponding ketone.

And finally, let’s look at this benzylic alcohol. Since this is a primary alcohol, I’ll turn it into the corresponding aldehyde with this Swern oxidation reaction and it’ll stop there.
