Hydroboration-Oxidation of Alkenes
Hydroboration-oxidation is an important method for converting alkenes into alcohols in a highly regioselective and stereospecific way. This reaction yields alcohols with the OH group on the less substituted carbon, and it’s also a strict syn addition—meaning both the new C–H and C–OH bonds point in the same direction in the final molecule. In this tutorial, we’re going to dive into the details of the hydroboration mechanism and common mistakes to watch out for on your exam.
Hydroboration-Oxidation Mechanism
As the name suggests, hydroboration-oxidation is a two-step process.
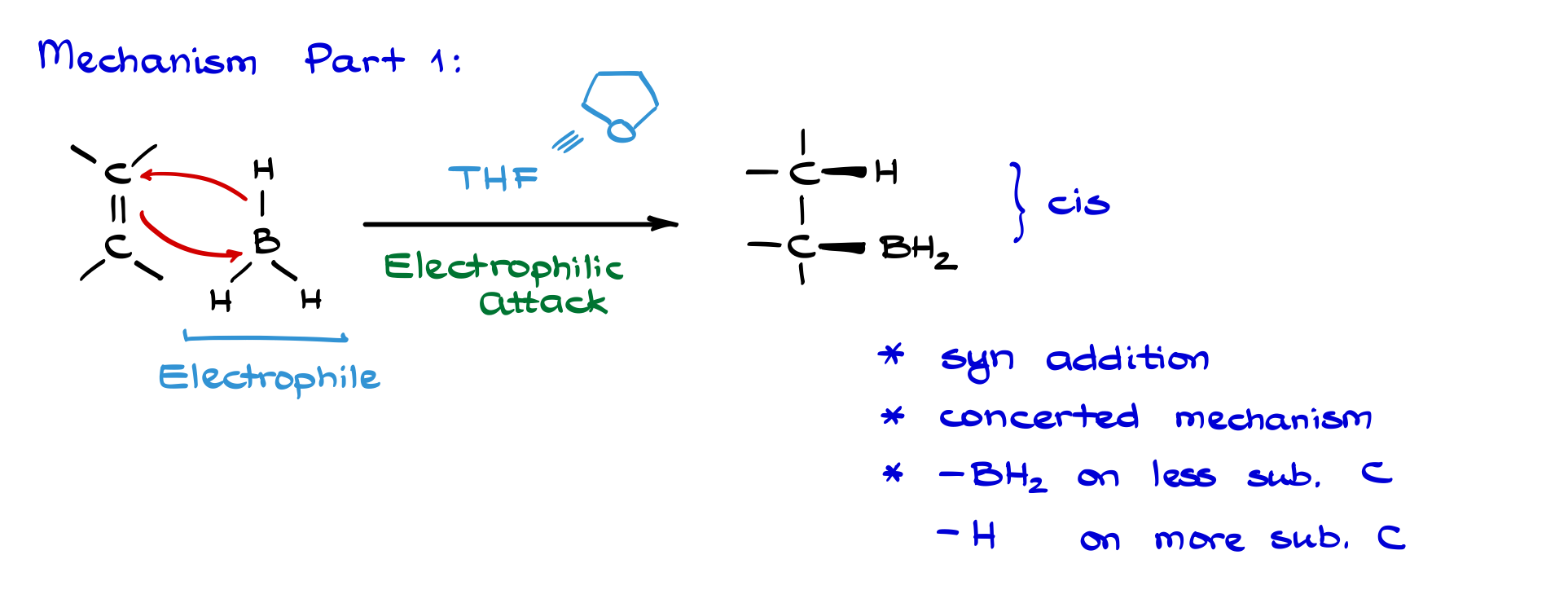
In the first step, we react our alkene with a borane molecule. So, here I have a generic alkene, and boron being an extremely strong Lewis acid pulls electron density from the alkene toward itself. Simultaneously, a hydrogen jumps onto one of the carbons. This is a concerted process, which means the whole thing happens in a single step, all at once. Because the borane acts as a Lewis acid, we classify this as an electrophilic attack on the alkene.
During this step, we form both a new carbon–hydrogen bond and a new carbon–boron bond. So, I end up bonding this carbon to a hydrogen and the other carbon to boron, giving a product that looks like this. Now, a few key points here: first, this is a syn addition. That means both groups we add (in this case, the hydrogen and the boron) end up on the same face of the molecule, so they’re cis to each other.
Another crucial detail is that this is a concerted mechanism—everything happens in one continuous motion. There are no intermediates in between the starting materials and the product of this step. I also mentioned this reaction is regioselective: the BH₂ ends up on the less substituted carbon, while the hydrogen attaches to the more substituted one. We’ll see exactly how that plays out in a real example after we finish this general overview.
A couple more notes: this reaction is usually done in the presence of THF, which stands for tetrahydrofuran. THF stabilizes BH₃ because BH₃ is such a strong Lewis acid that it can’t exist by itself—it tends to dimerize or polymerize. When it clumps together like that, it’s not reactive enough, so complexing it with THF keeps it active and ready to react.
Another interesting aspect is that this addition can occur up to three times, depending on how bulky the alkene is. The boron atom can bond to up to three alkyl groups, although in practice you usually get a mix of products where boron has one to three alkyl groups attached. For simplicity, though, we’ll pretend it only happens once and move on to the oxidation step.
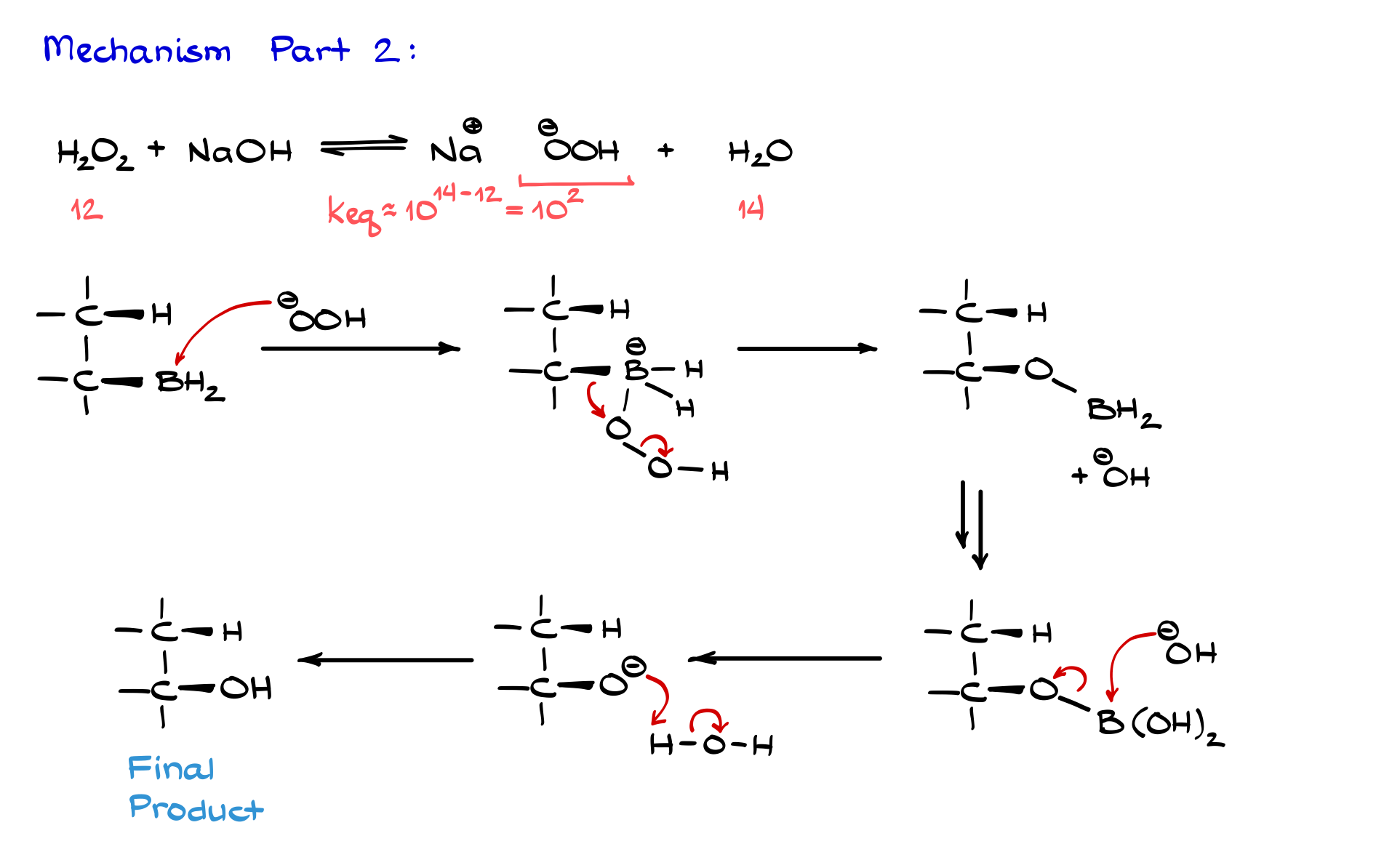
The oxidation is typically done with hydrogen peroxide in the presence of a base, usually sodium hydroxide or sometimes potassium hydroxide. When hydrogen peroxide and sodium hydroxide are in solution together, an acid–base equilibrium forms, producing Na⁺ with OOH⁻ (the peroxide anion) and water as a byproduct.
If we think about this in terms of acid–base chemistry, the pKa of hydrogen peroxide is around 12, while the pKa of water is 14 (or 15.7 depending on your textbook). That means the equilibrium constant for this reaction is about 10 to the power of (14 minus 12), so around 10². So, this reaction favors formation of -OOH, and that’s important because -OOH is what actually oxidizes the boron-containing compound from the previous step.
So, we take our boron intermediate and react it with -OOH. The oxygen attacks the boron because boron, being such a good Lewis acid, gladly accepts those electrons. We form a species where boron is bonded to the alkyl group, a couple of hydrogens, and the OOH group. Now, we have a formal negative charge on boron.
Something interesting happens next: the electrons in the carbon–boron bond migrate toward the oxygen, breaking the oxygen–oxygen bond and releasing OH⁻ into the solution. This means that the oxygen inserts itself between the carbon and the boron—so the electrons that were in the carbon–boron bond are now in a carbon–oxygen bond.
The same thing happens between boron and its hydrogens—we insert oxygens there too. To keep it simple, I’ll skip redrawing every step and jump straight to the final intermediate, which looks like this: boron bonded to multiple OH groups, plus the alkoxide attached to carbon.
At this point, because we have plenty of OH⁻ floating around and no more peroxide left, OH⁻ slowly knocks the alkoxide off the boron. In reality, this step is more complex, but within the scope of our course, it’s fine to use this shortcut. Once we have the alkoxide, water in the solution provides a proton, giving us the final alcohol.
One important thing I glossed over earlier is the stereochemistry at the carbon bonded to boron. I first focused on the electron flow, but let’s revisit that. We start with the boron bonded in a specific orientation—say, looking at us. When the oxygen inserts itself between boron and carbon, the stereochemistry stays exactly the same. So, if the boron is pointing toward us, the oxygen does too. This retention of configuration is a big deal: the stereochemistry of the carbon–boron bond is exactly the same as the final carbon–oxygen bond. If boron was pointing at us, oxygen points at us; if it was pointing away, so does the oxygen. This becomes very important with real molecules.
Hydroboration-Oxidation of Alkenes Examples
So, let’s look at an example with my trusty friend, one-methylcyclohexene.
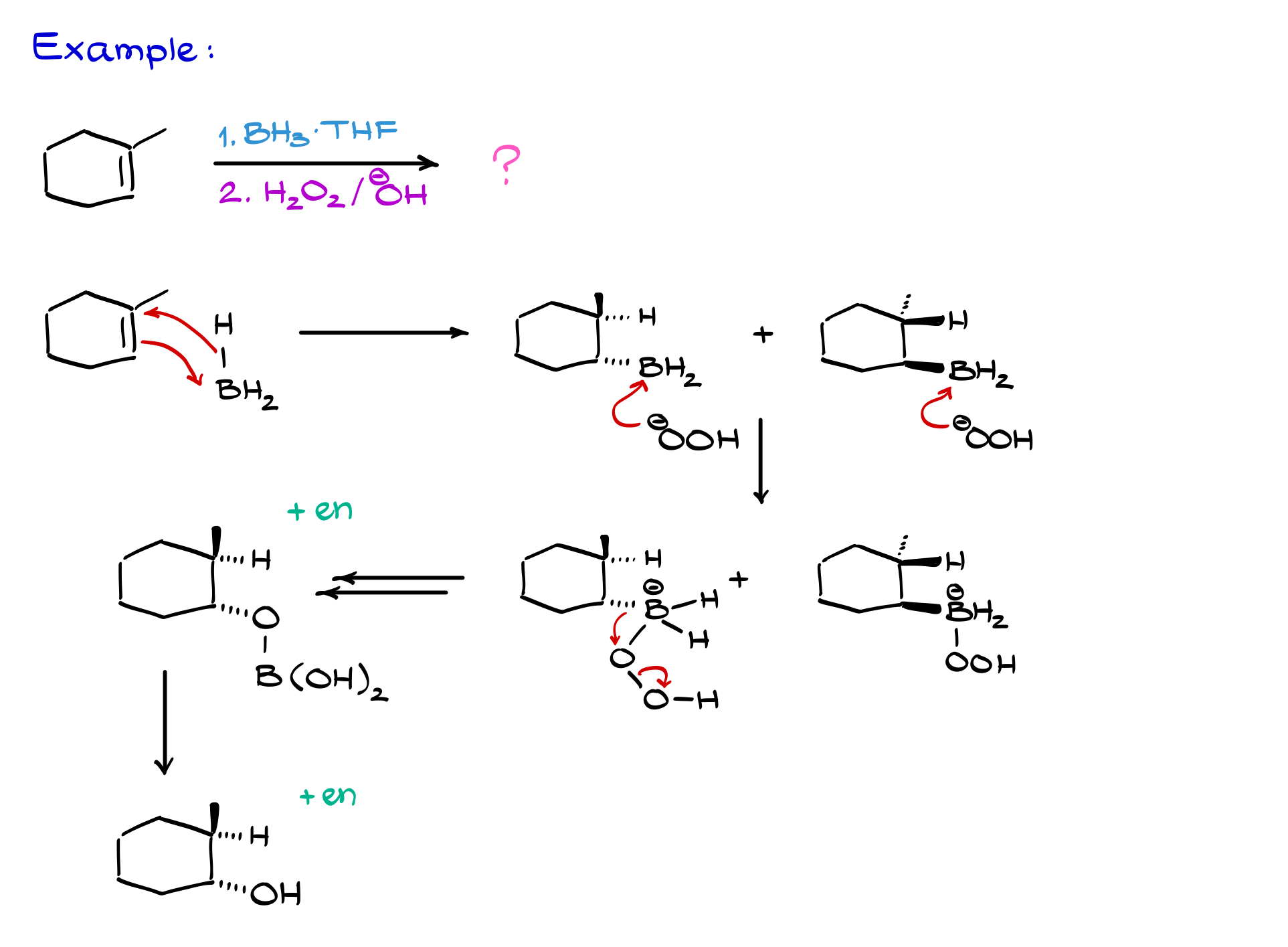
In the first step, boron adds to the less substituted carbon while hydrogen goes to the more substituted carbon. I orient the molecule to make the mechanism clear: the double bond attacks boron, the hydrogen jumps onto carbon, and we get a product like this.
Since boron can attack from the back face or the front face, we get a pair of enantiomeric intermediates in this first step. Next, we move to the oxidation step. I’ll use the shortcut: OOH⁻ attacks boron, we do our bond shifts, and end up replacing BH₂ with OH. So, for one enantiomer, the final alcohol looks like this, and for the other, it’s the mirror image. Because this is a strict syn addition, the hydrogen and OH are on the same side—both dashes here, or both wedges for the enantiomer.
Here’s a common trick: sometimes, the hydrogen is implicit and not shown at all. So, you might see a product with a methyl group on a wedge and an OH on a dash, and think it’s wrong because you know the addition is syn. But remember, the hidden hydrogen is right there—don’t let that trip you up on an exam.
Another Example
How about another example?
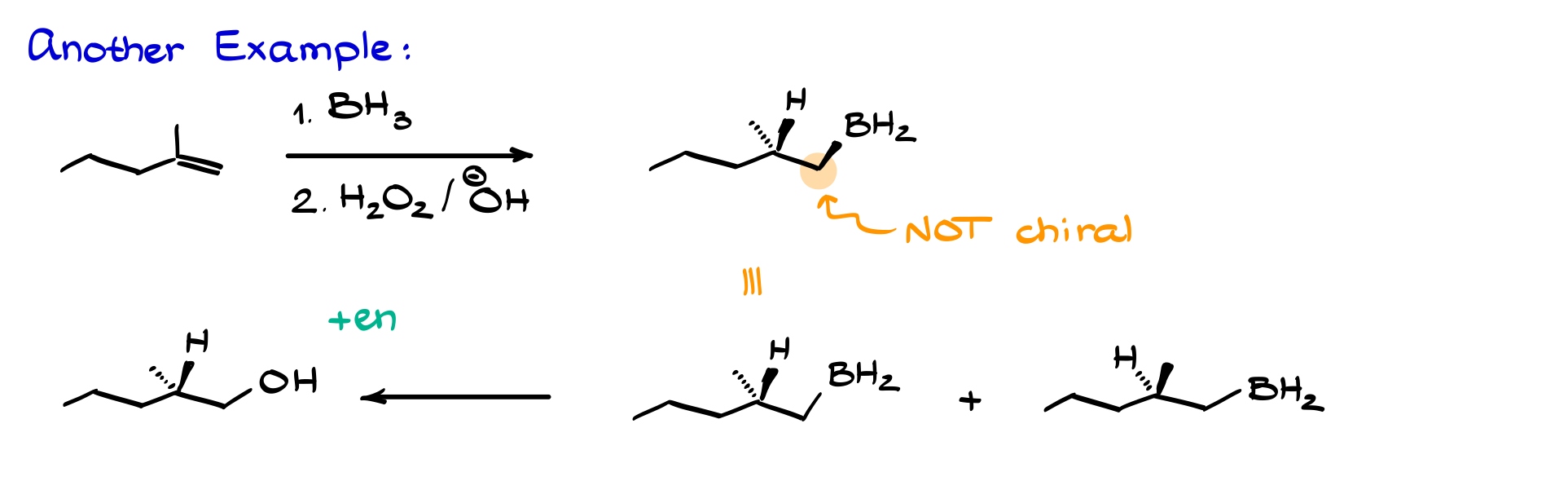
Let’s say we add boron so that hydrogen and boron attack from the front face—both looking at us—and the methyl group is pointing away. The carbon with the boron isn’t chiral, so we don’t even need to draw a wedge or dash for boron; a plain line works just fine. For the enantiomer, just flip the stereochemistry on the chiral carbon so the hydrogen points away and the methyl group looks at us. Then, do the oxidation step, swap BH₂ for OH, and you have your final product plus its enantiomer.
Tricky Example
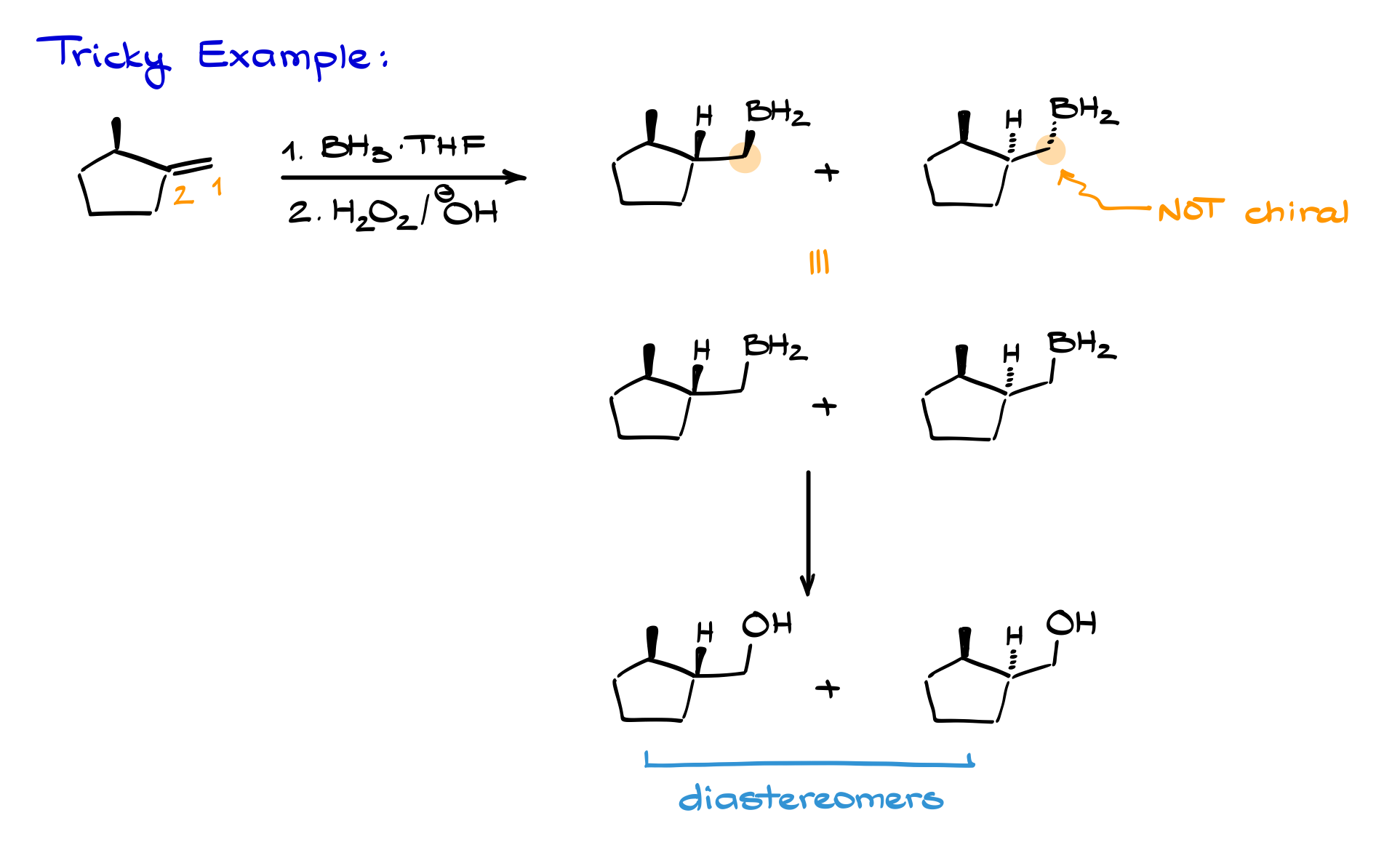
One more tricky case: suppose we predict the product for a reaction mechanistically. Boron adds to the less substituted carbon, hydrogen to the more substituted carbon. We get two possibilities. But here’s the catch: these two products aren’t enantiomers—they’re diastereomers because the molecule might already have other stereocenters or symmetry breaking the mirror image relationship. So, never assume products are always enantiomers—check carefully.
As you can see, these reactions have lots of places where things can go sideways if you’re not paying attention to the mechanism and stereochemistry. I know I sound like a broken record, but seriously—practice these until you can do them in your sleep. Understanding this reaction inside and out will save you big points on your exam.

I love you!!
I’m glad you’re liking my content! 😃