32. How to Add an Ethyl Group to an Alkene
In this tutorial I wanna go over this synthesis where we start from methylcyclohexene and we end up adding an extra chain to the second carbon of the double bond.
So, typically, when we’re thinking about C–C bond formation, we usually go straight to the Grignard reaction, which often gives an alcohol as the product. But here, obviously, we don’t end up with an alcohol. Instead, we’ve got an alkene. And alkenes can be easily made via a simple E1-style elimination.
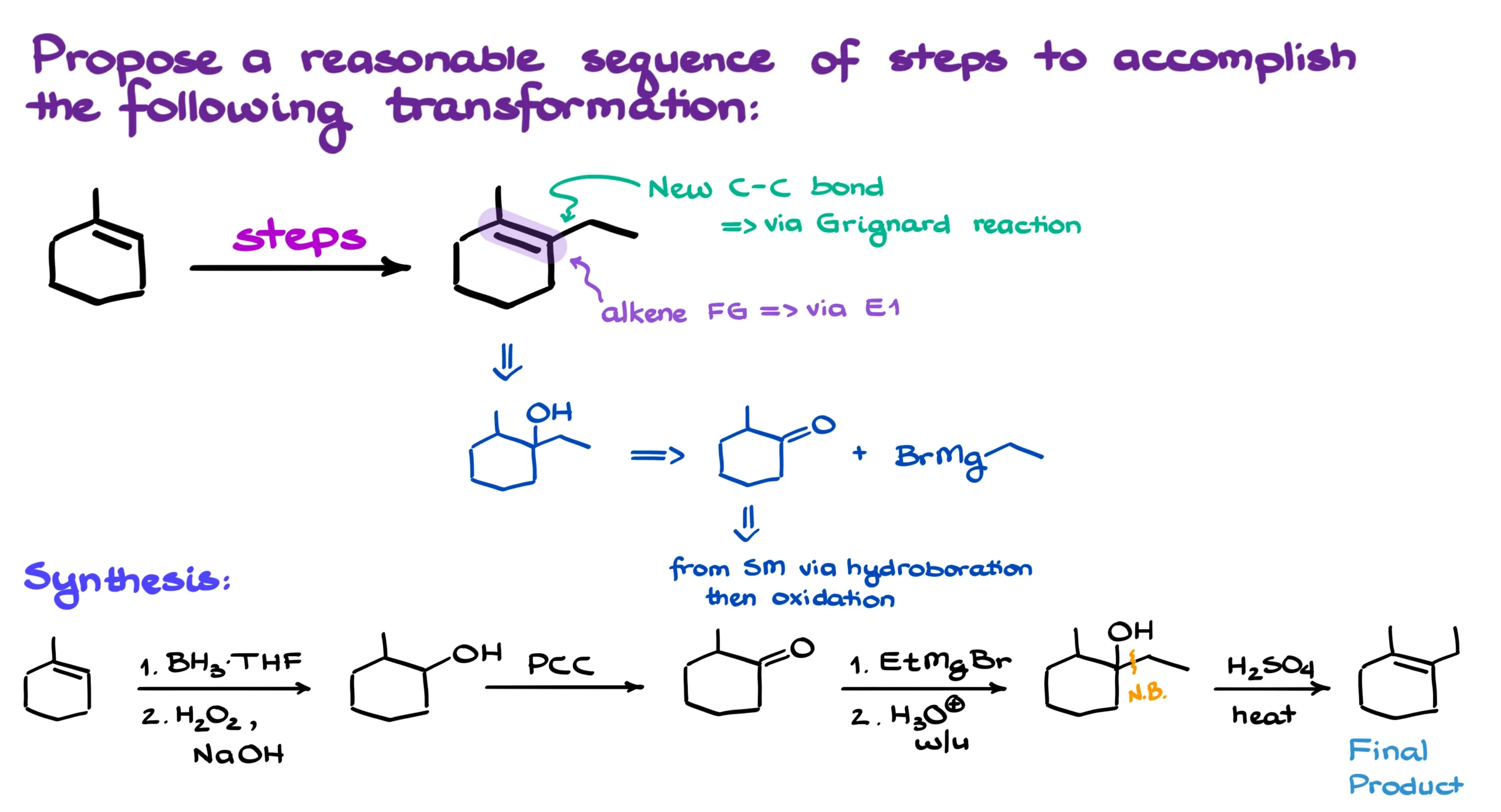
Which means that the molecule right before the last step must’ve been this guy.
Now, the Grignard reaction requires a carbonyl group, so the reagents that made my alcohol would’ve been something like methylcyclohexanone and ethyl magnesium bromide.
And of course, we can make that cyclohexanone intermediate pretty easily from the starting material in a couple of steps—first doing hydroboration-oxidation, then oxidizing the resulting alcohol.
So now that we’ve mapped out our synthesis and we know the key reactions, let’s piece it all together!
I’m going to start by redrawing the starting material and running through the hydroboration-oxidation, which gives us the corresponding alcohol. The stereochemistry doesn’t really matter for our purposes here, but I still wanna point out that this is a syn-addition reaction, and it gives the anti-Markovnikov product.
Once we’ve got our alcohol, we’ll oxidize it to the ketone. Since it’s a secondary alcohol turning into a ketone, we can choose pretty much any oxidizing agent we like without worrying about over-oxidation. I’ll go with PCC—because why not!
And from there, you already know the drill: Grignard comes next, and then we do the elimination to wrap up the synthesis and get our final product.
Of course, this isn’t the only way to pull off this synthesis. Another cool route that comes to mind is opening up an epoxide with a Grignard reagent. But honestly, I’d love to hear your thoughts. Got any interesting ways you’d approach this synthesis? Let me know in the comments below!
