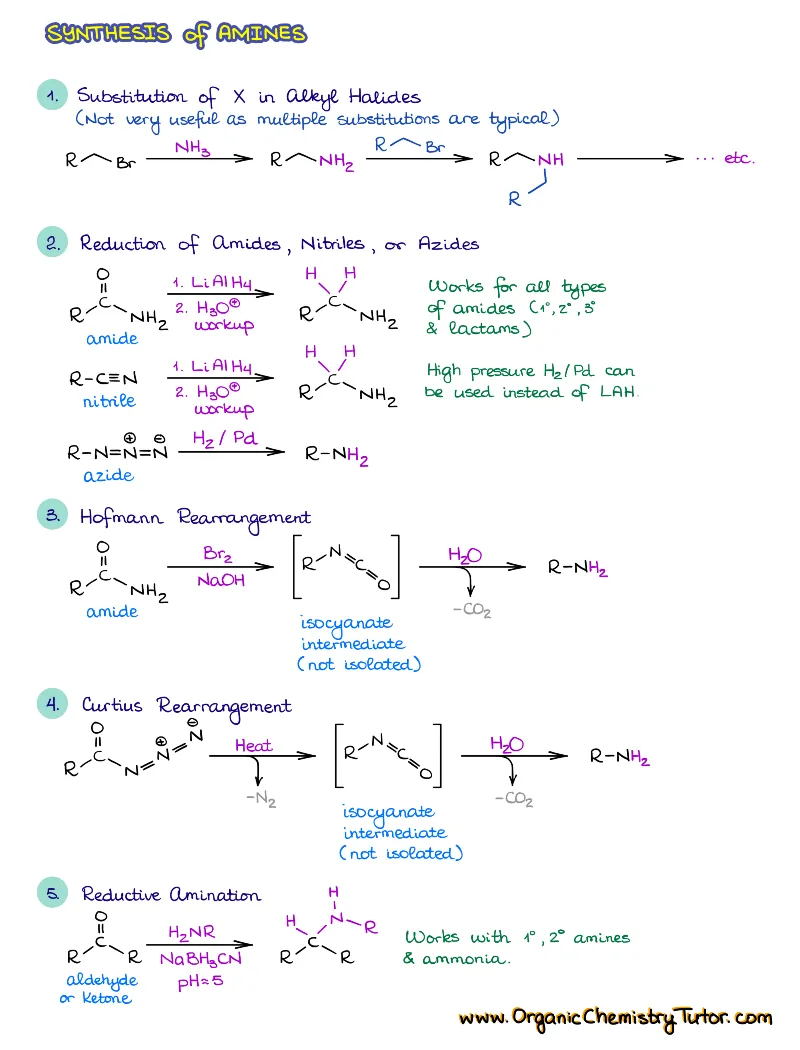
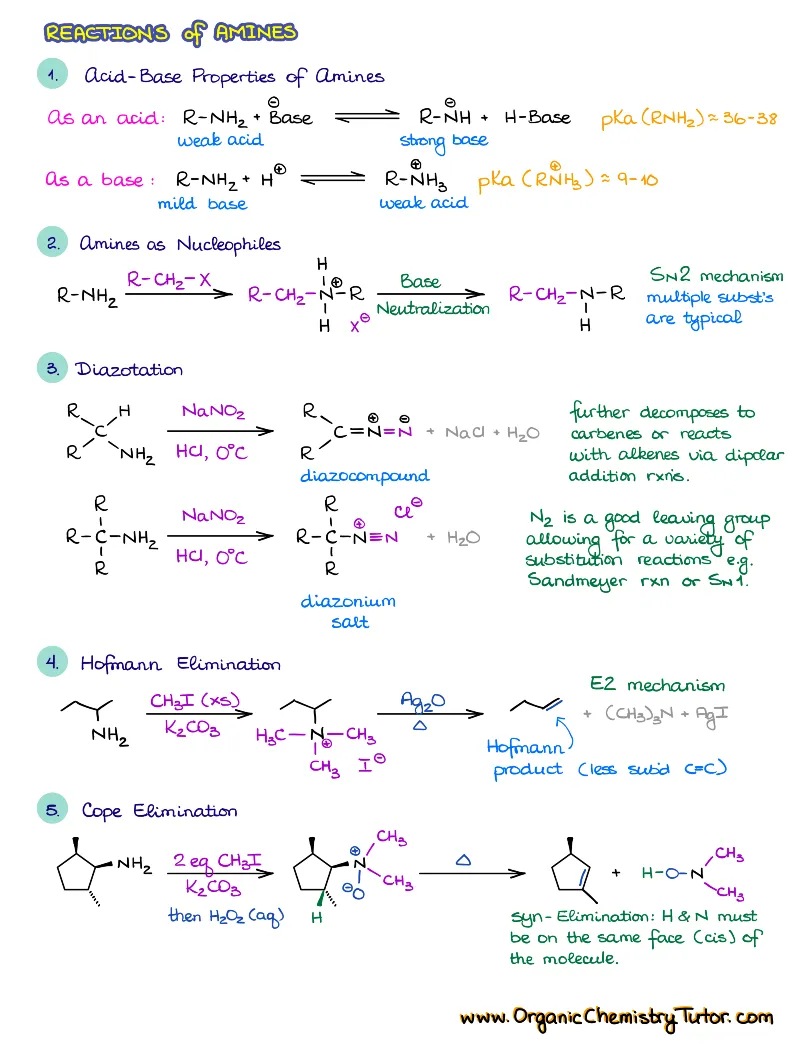
Are you looking for a complete set of organic chemistry summary notes? This is exactly a set like that! With over 300 pages of notes, summaries, examples, tips & tricks, and detailed mechanisms, plus the complete access to all members-only organic chemistry content on the site, this is truly one of the most comprehensive collections you can get to help you ace your next exam!
Save a ton with this bundle offer and get all the organic chemistry notes, summaries, and cheat sheets, plus all members-only content on the website! That’s more than 300 pages of meticulously hand crafted, designed and color-coded notes! This is one of the most comprehensive collection of organic chemistry notes available on the Internet and it is constantly growing! So, stop wasting time focusing on frantic note taking and start learning!
This collection currently includes:
- Bonding and resonance (from VSEPR theory, to common patterns, to types of resonance, etc.)
- Detailed notes on nucleophiles, electrophiles, substitution, and elimination mechanisms
- Notes on the acid-base chemistry with examples of how to use the pKa table and acid-base equilibrium in organic chemistry
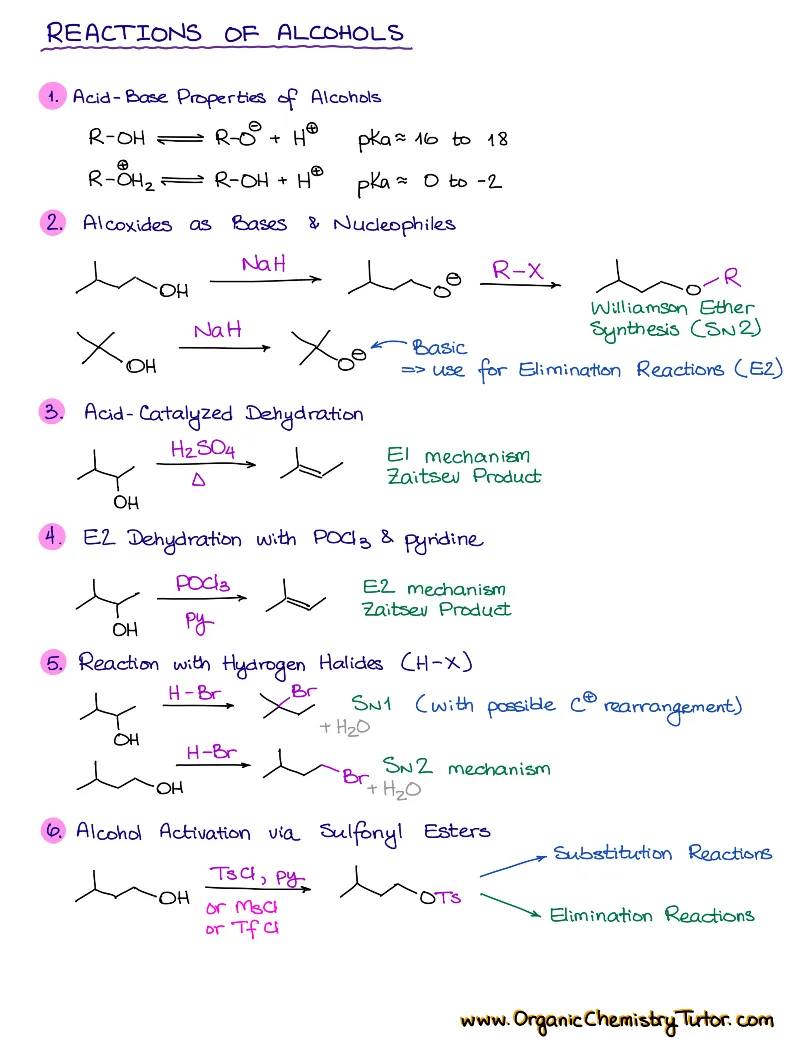
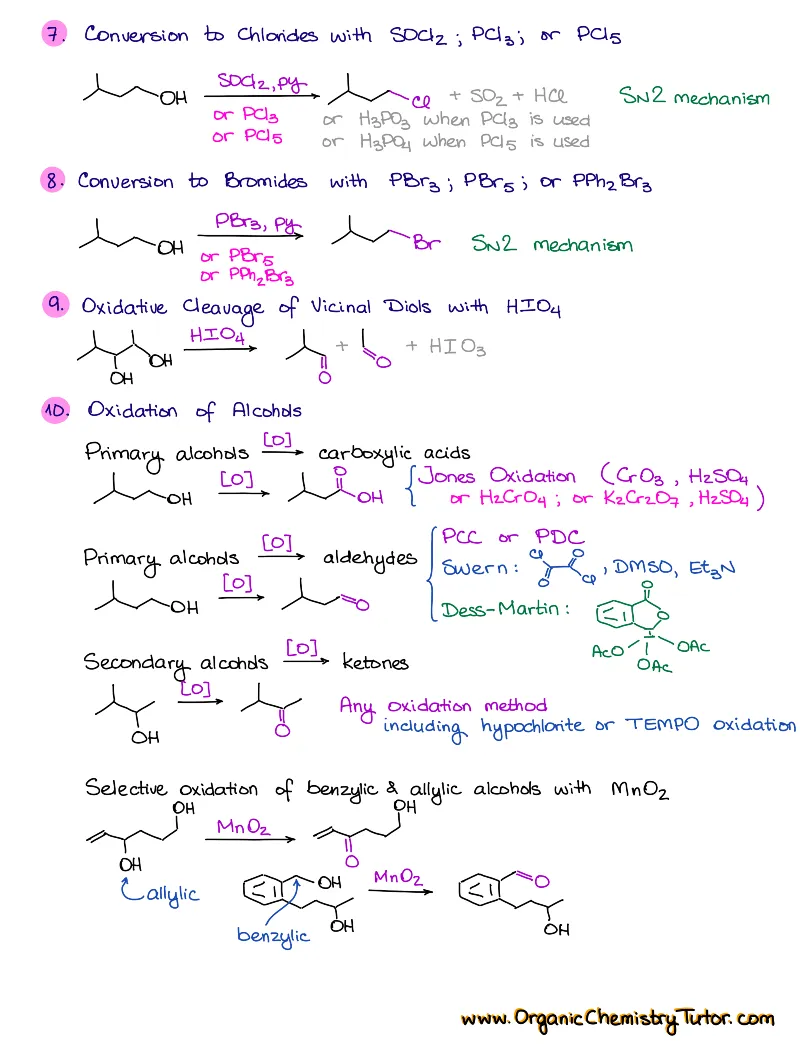
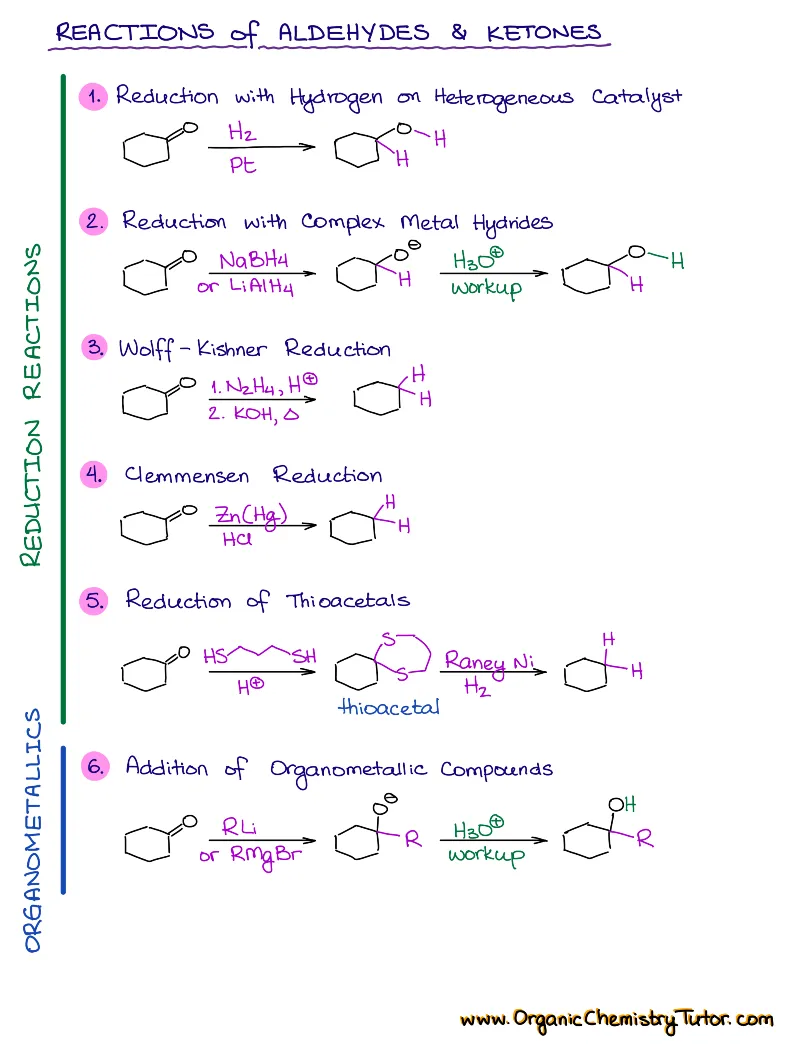
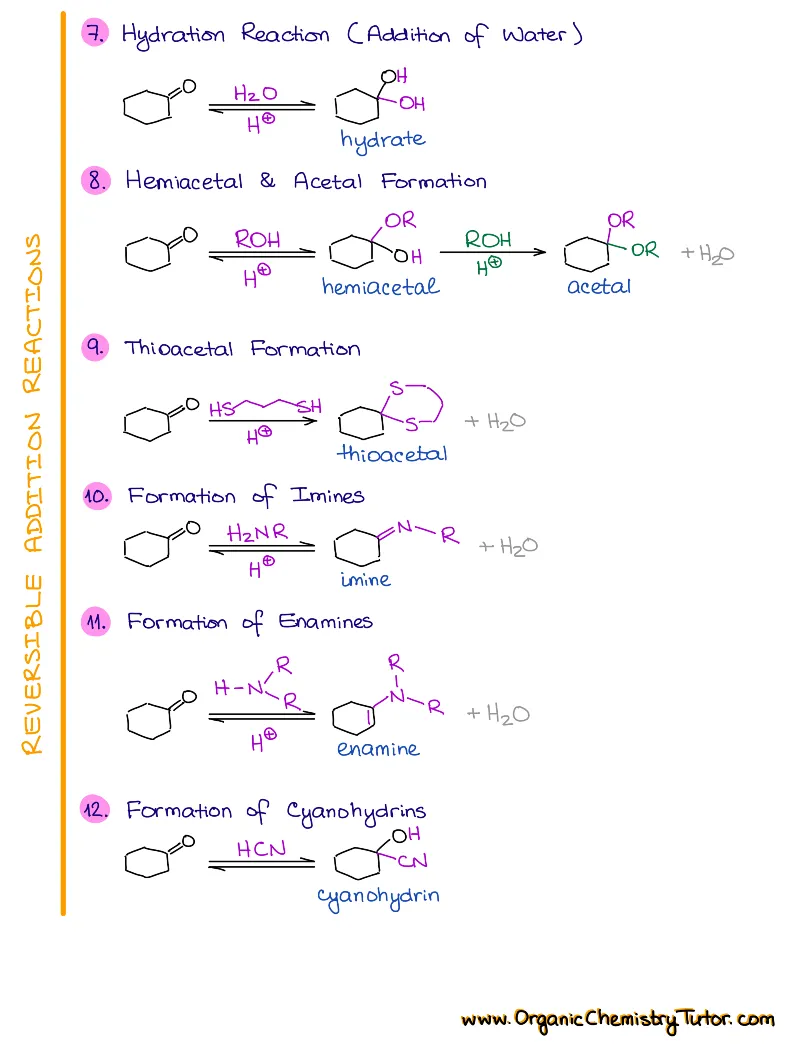
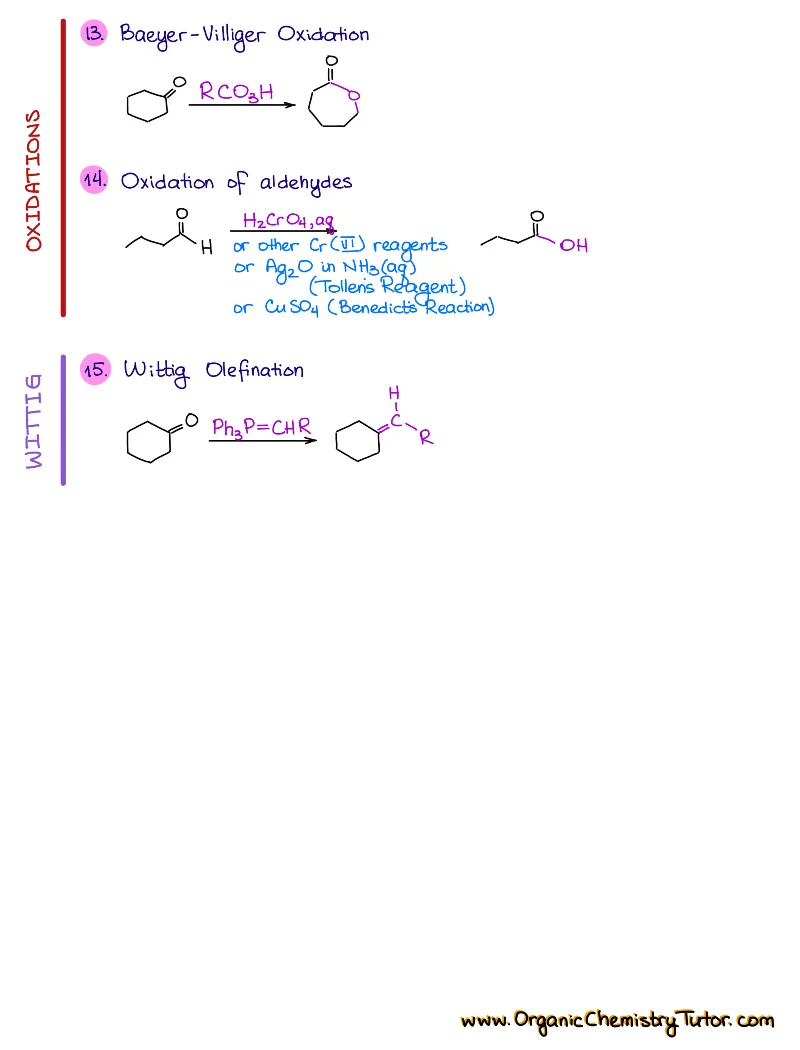
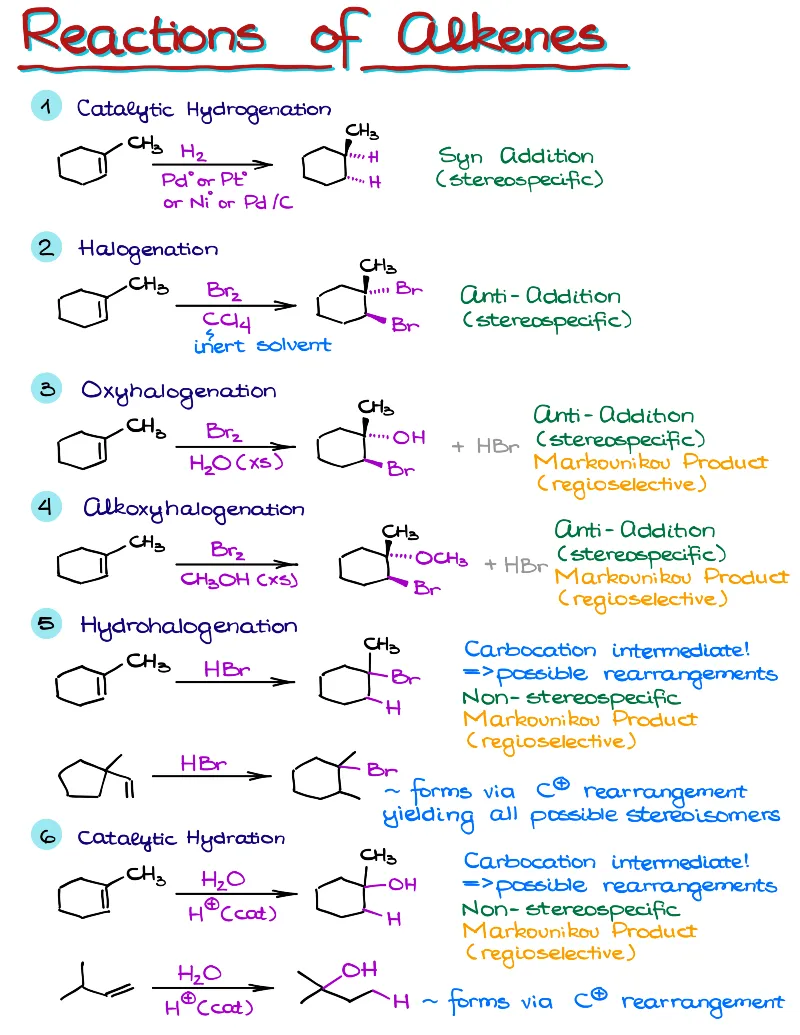
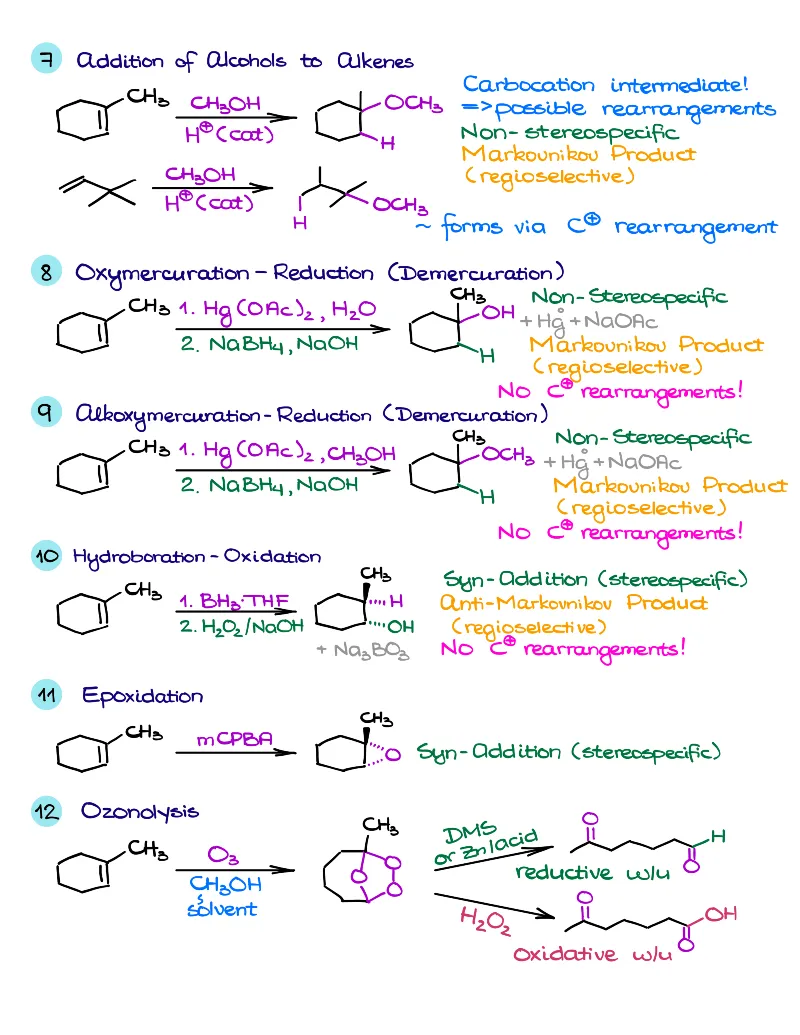
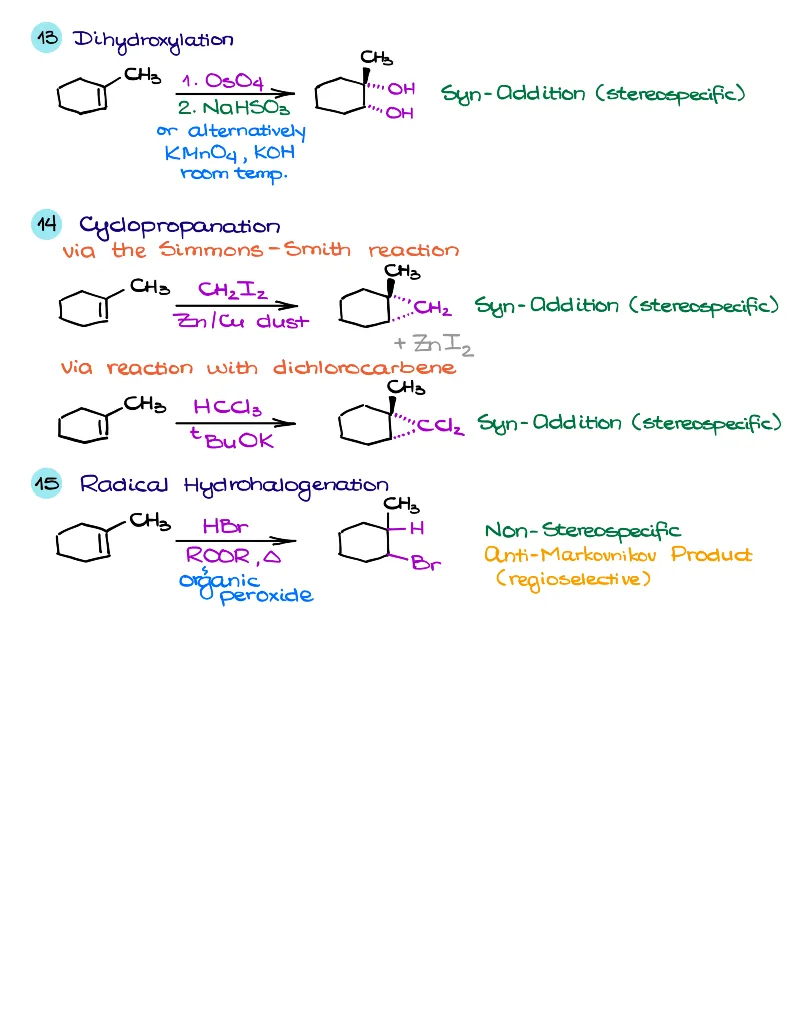
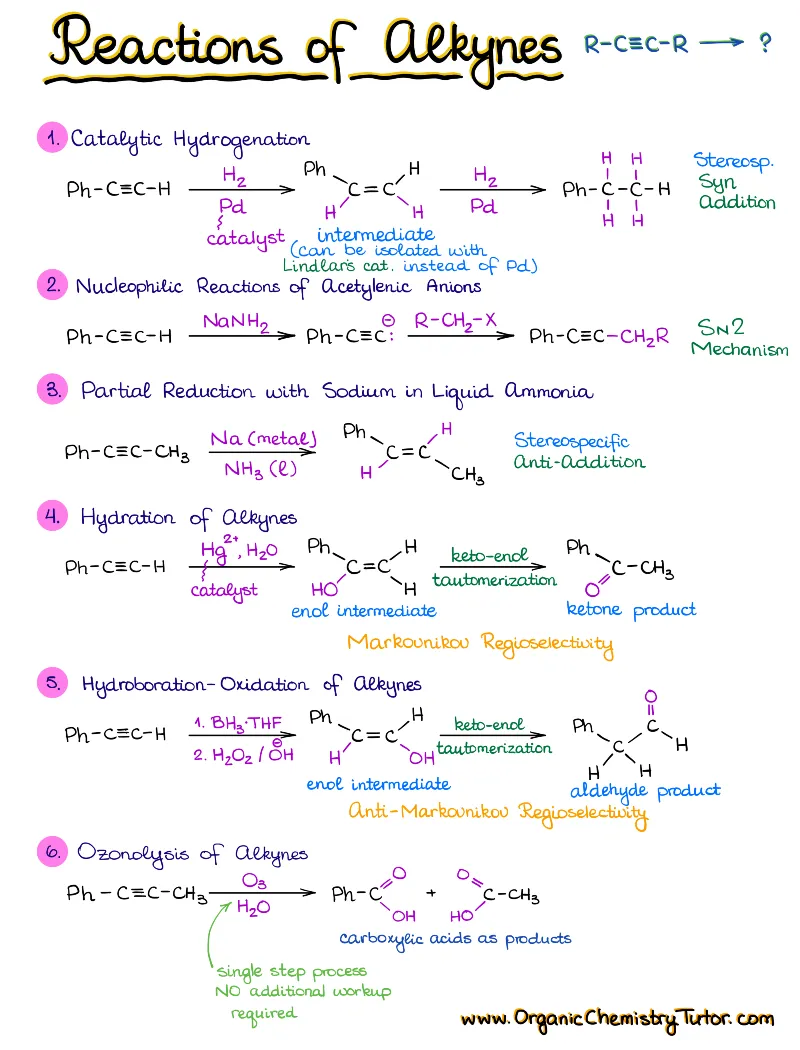
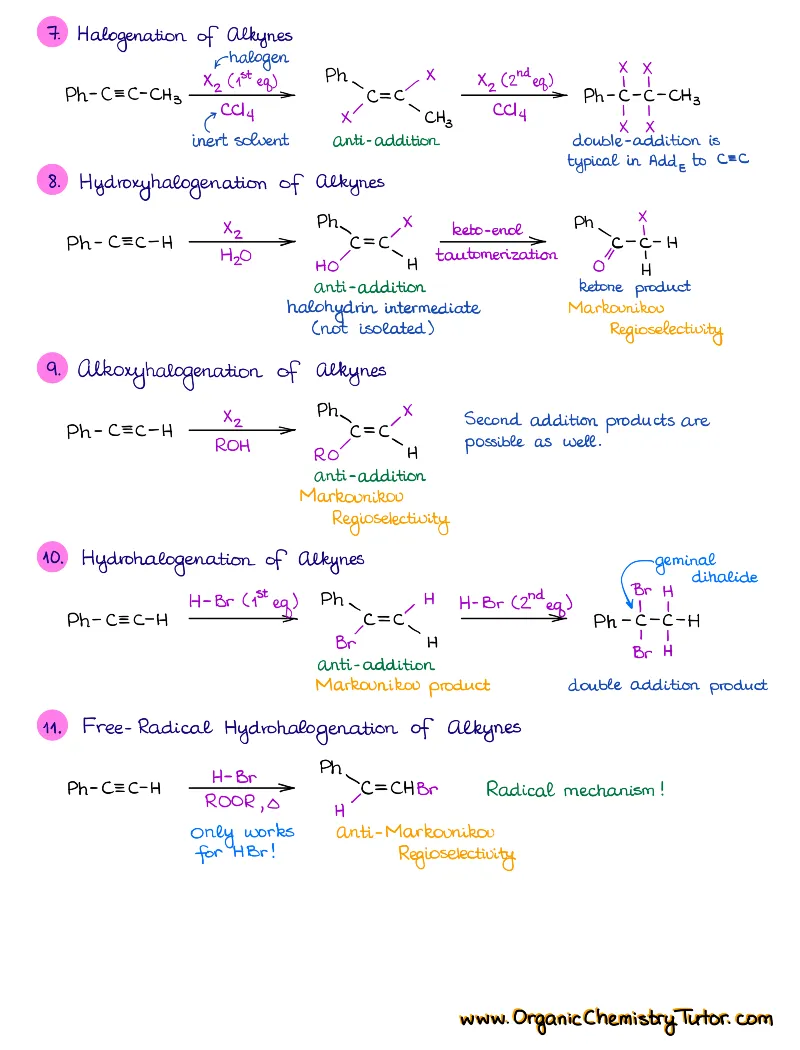
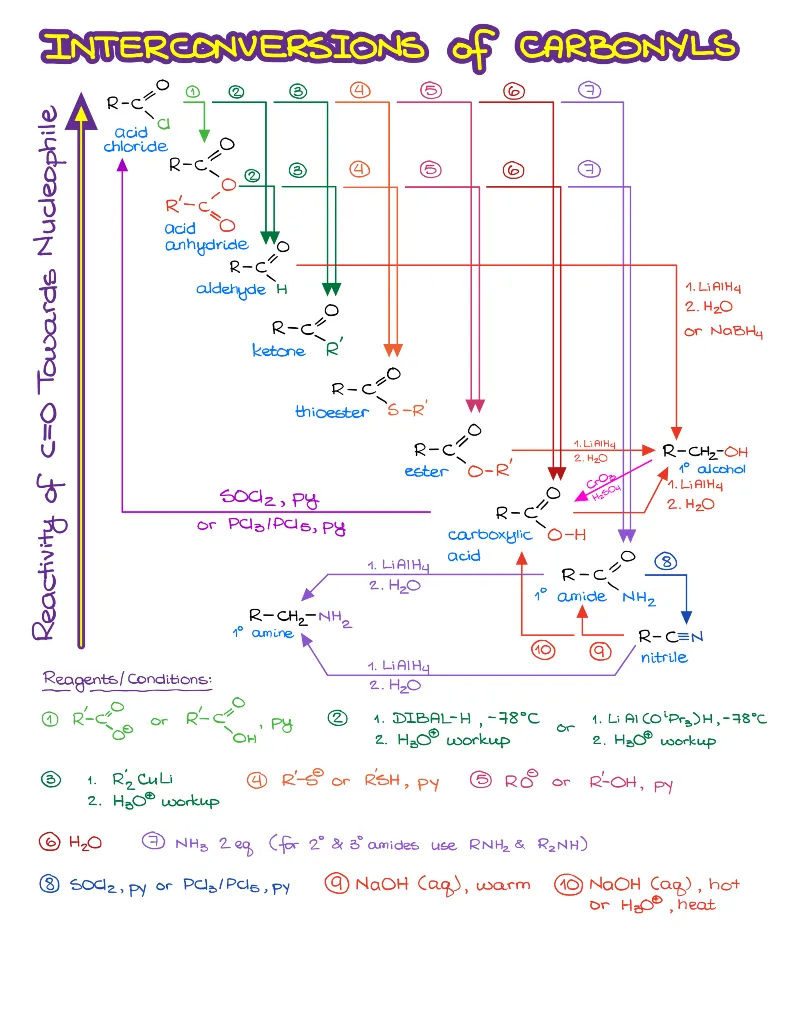
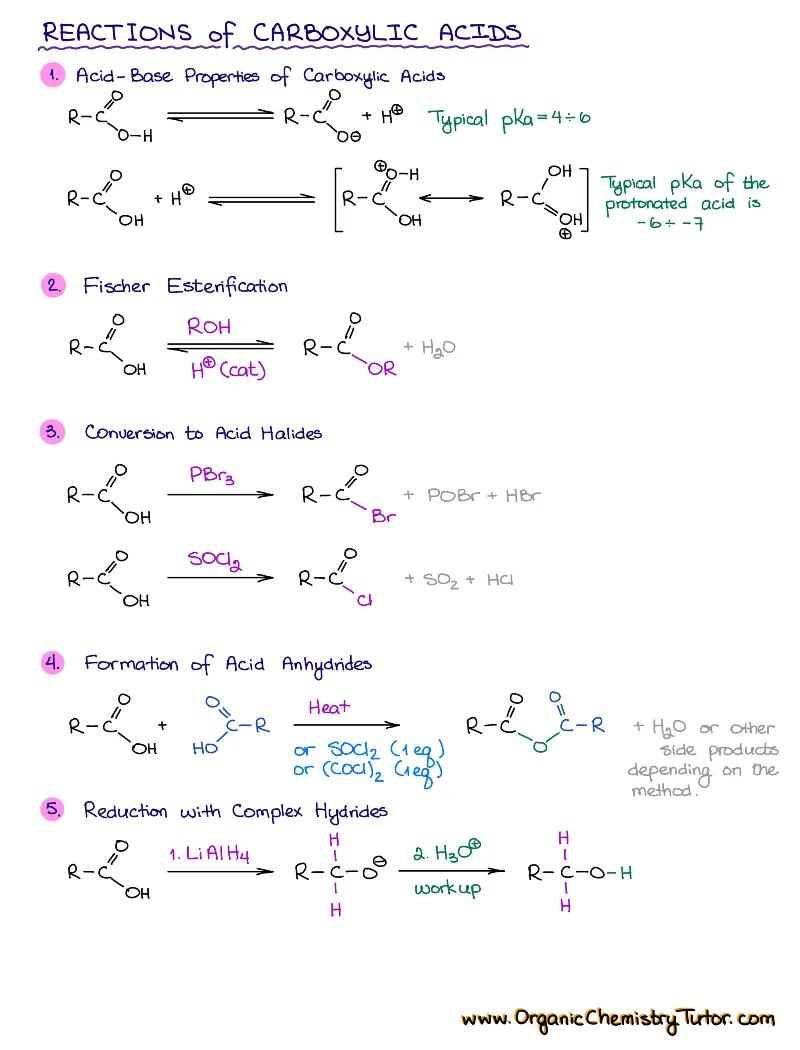
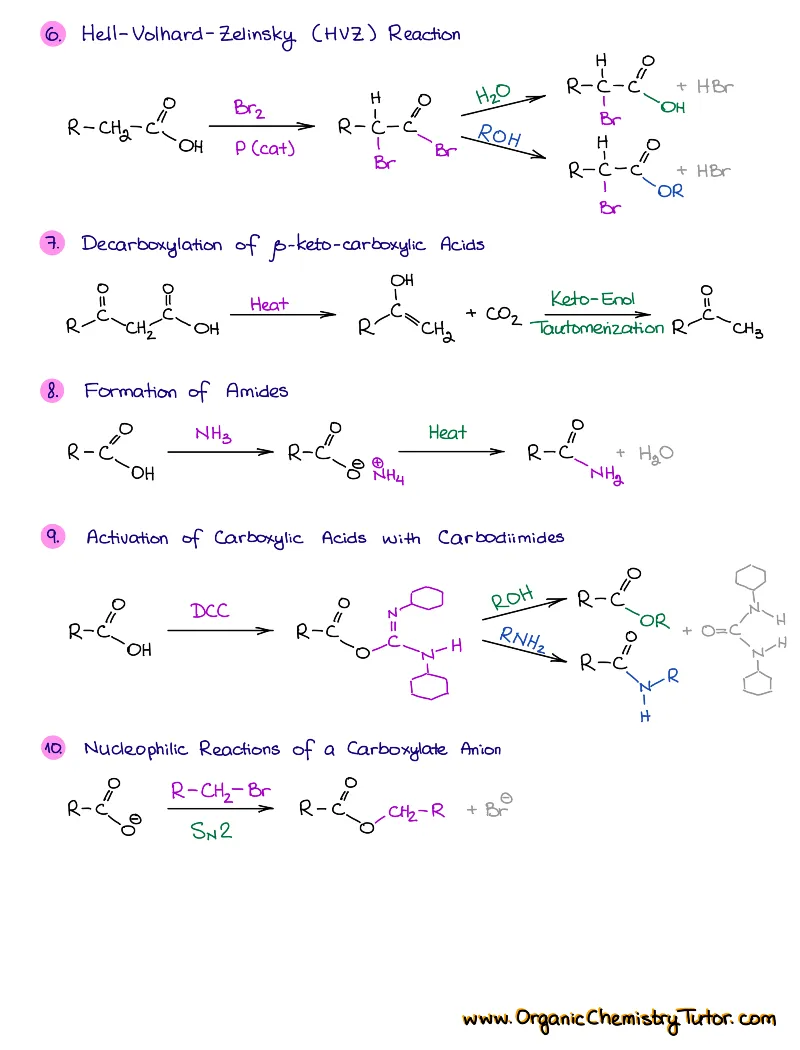
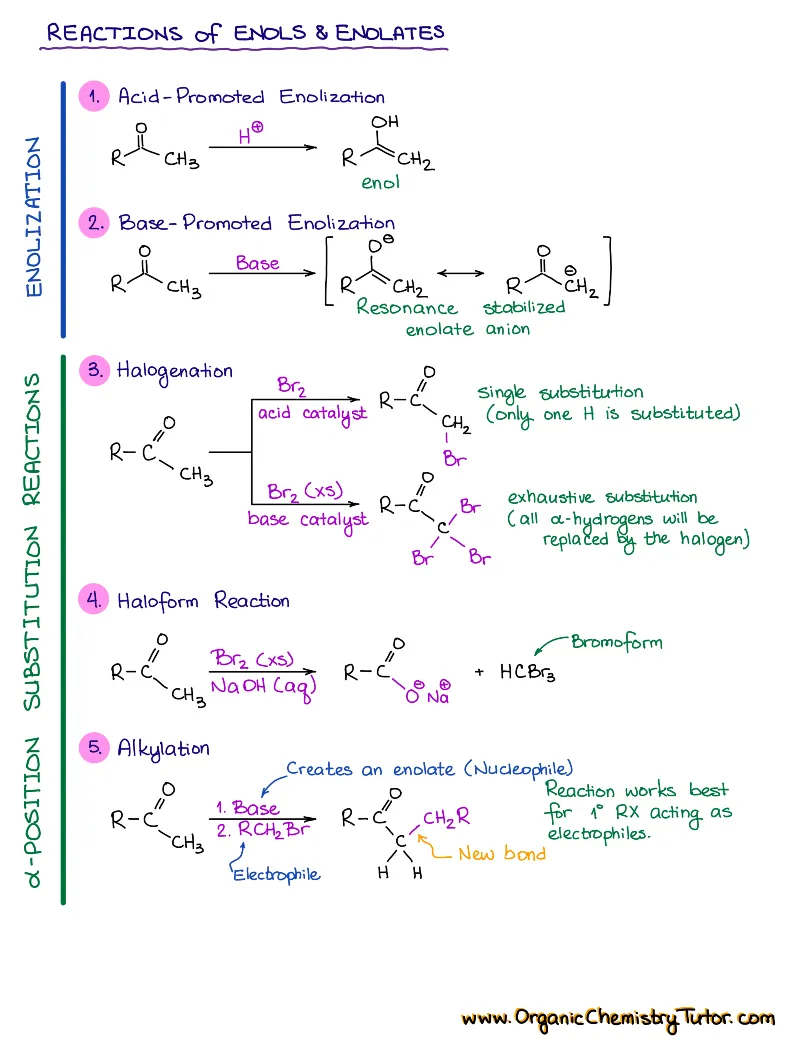
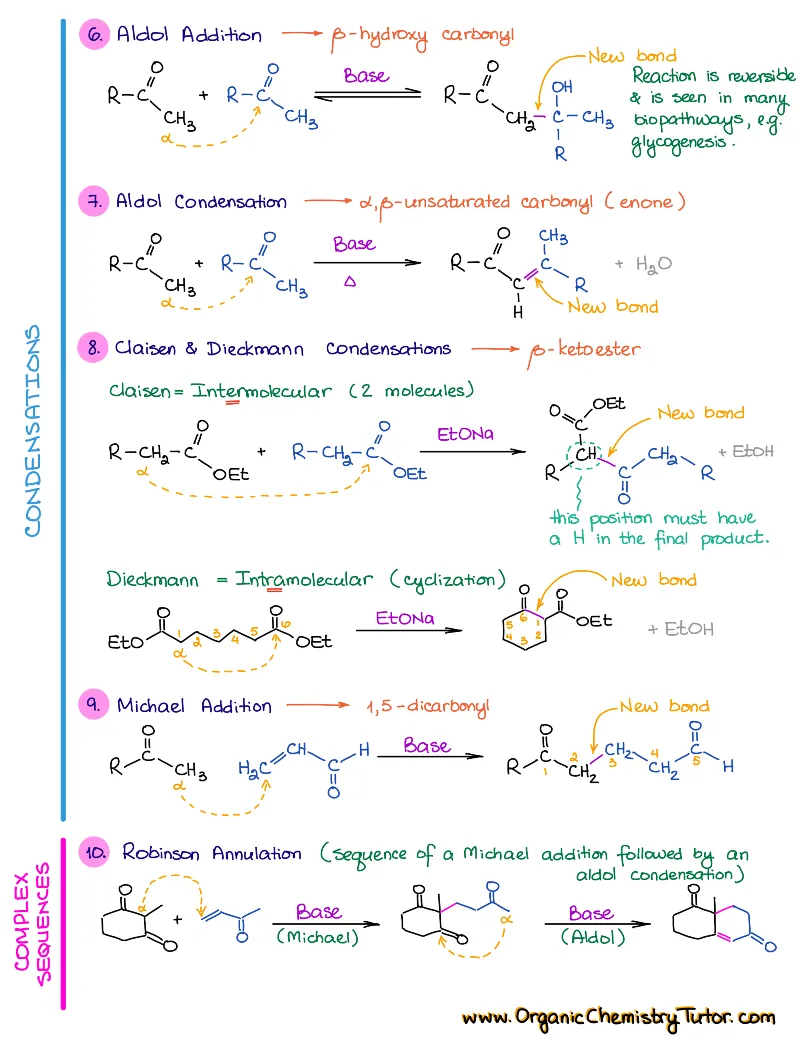
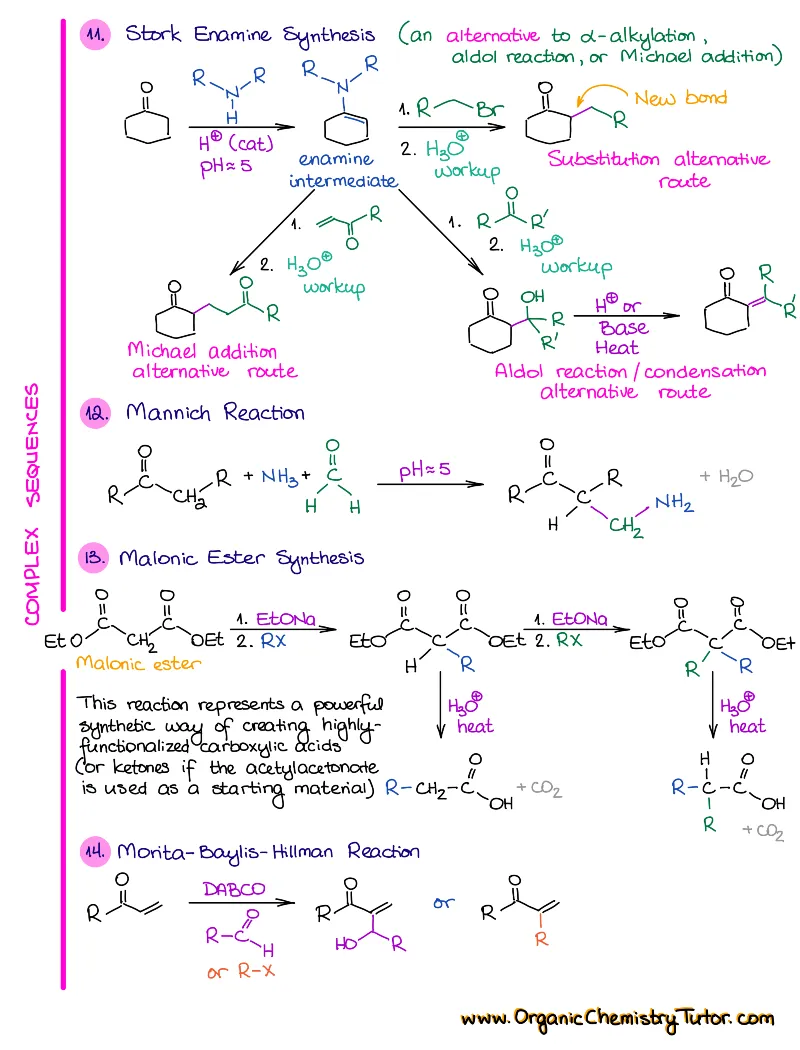
- Reactions of alkenes, alkynes, alcohols, aromatic compounds, carbonyls (aldehydes and ketones), carboxylic acids, enols and enolates and more with detailed mechanisms and explanations!
- Stereochemistry including the chair conformations, enantiomers, diastereomers, and meso compounds
- Spectroscopy with a details step-by-step guide to solving spectra
I constantly update, expand, and tweak my notes to make them the ultimate guide to your success in organic chemistry! Keep in mind that access to downloadable materials is non-refundable. After all, what’s been downloaded, can’t be undownloaded 😉
PDF Library +
Semester Pass
$127.97 one-time charge
Charged once
No recurring payments
How are these organic chemistry notes different?
I carefully design my summary notes based on how students use them. When you write your notes, you probably start a new topic on a new page, right? You probably don’t try to jam as much stuff as you can on a single page? I approach my notes with the same idea: each topic or a reaction is its own page (or two if that’s a long mechanism). This makes these notes perfect from printing out and adding to your organic chemistry binder. You can only print the pages you want and you’ll never have to worry about awkward breaks.
I also use a consistent color coding scheme throughout my notes, so it is very easy to follow them. For instance, I only use red for curved arrows in the mechanisms. This way, you can always easily skim the mechanism to get the feel for the electron flow that will be visually distinct from the rest of the notes!
How these notes are organized?
The notes are organized by the chemistry of each functional group. Plus, I have dedicated sections for spectroscopy, stereochemistry, and substitution & elimination reactions. They are also written in such a way as to allow you to pull separate pages apart and compile your own study or review set. This also makes it easy to add those to your own notes.
Many students who bought these notes told me that they’ve uploaded them to their favorite note taking app like GoodNotes or Notability and then pulled the pages they needed for the review when they needed them adding their own class notes in between or adding these notes to their class notes making a comprehensive study guide. The notes are intended to be “pulled apart” and re-organized in a way that works for you and your course.
I can take my own notes, why do I need these?
You absolutely can you you should take your own notes! When you’re rewriting or reorganizing your own chemistry notes, however, you’ll be facing the challenge of condensing, reworking, and cleaning up your notes into a concise summary that you can use later on for reviewing. This takes time. And I kid you not, this takes hours if not days out of your life! Hours and days you could’ve been studying or practicing problems! I’ve done this work for you and I put my 20 years of teaching experience into organizing these organic chemistry notes into the most productive form for studying and review. For each topic I always start from the general overview, followed by the details and then, examples. This is a research-proven way to study chemistry that I embody in my summary notes.
Will these notes help me study?
Absolutely!
So far, I’ve told you a lot what these notes are. But let me tell you what these notes are not! They are not a magic pill that will solve all of your organic chemistry problems without you putting any effort in. I’ve purposefully combined these notes in a way to give you all the information you need about chemistry of each functional group without sticking to any specific textbook.
Unlike a textbook, my notes do not repeat the same information multiple times. But I still have it all. So, instead of 1500 pages that you have in the textbook, I’ve managed to condense the entire course to ~300 pages that you can use to stitch that perfect review set you need for a midterm, final, or just a class material review!
I constantly work on my organic chemistry notes and update them based on your feedback. I also regularly add new notes and new topics to existing sets. Is there a specific set you want to see in this collection? Just let me know and I’ll add it to my “to do” list.
Below are just some examples of the notes and pages you’re going to see in the Resource Library. Obviously, I cannot give you a full preview, but it should give you a pretty good idea of what the Resource Library is all about.
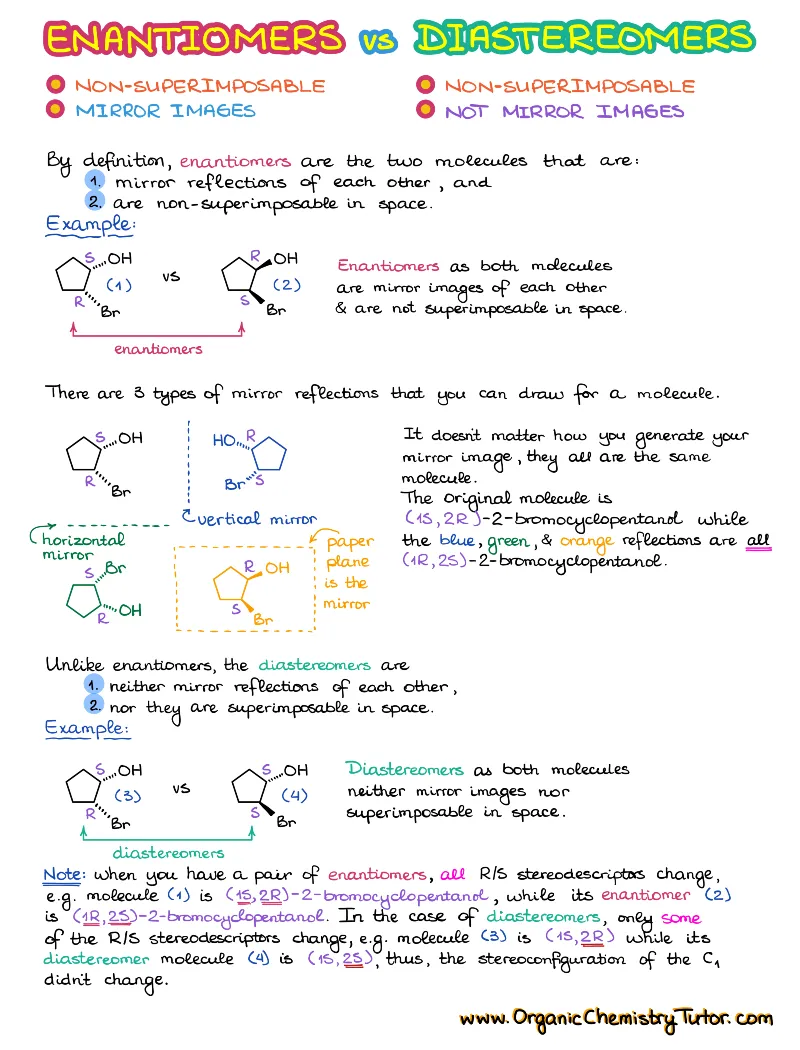
Stereochemistry
Stereochemistry is a fundamental topic in organic chemistry. This is a detailed set of notes going over the most important topics in stereochemistry to help you ace your exams!
What’s Included
- Types of Isomers
- Stereochemical Drawings
- Meso Compounds
- Newman and Fischer Projections
- Cahn-Ingold-Prelog (CIP) Priority Rules
- Stereochemical Descriptors (stereodescriptors: R/S, E/Z, D/L)
- Stereochemical Description of Reactions
- Stereochemical Resolution of Enantiomers
- Optical Activity and Optical Activity Calculations
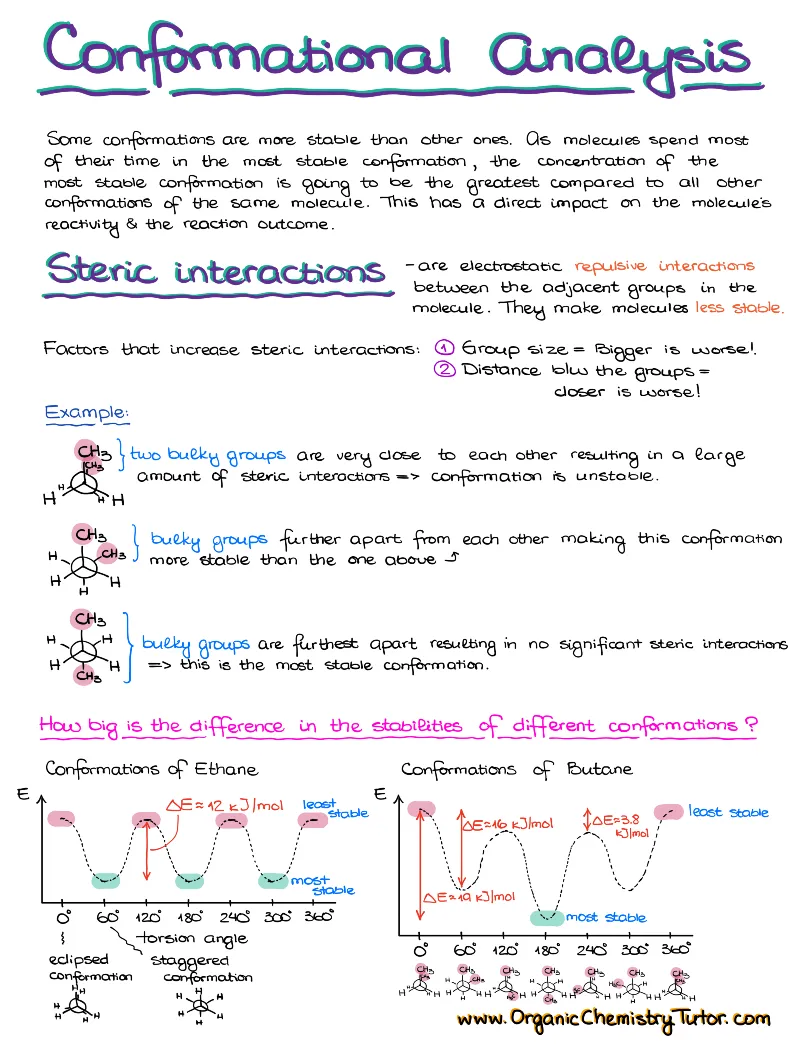
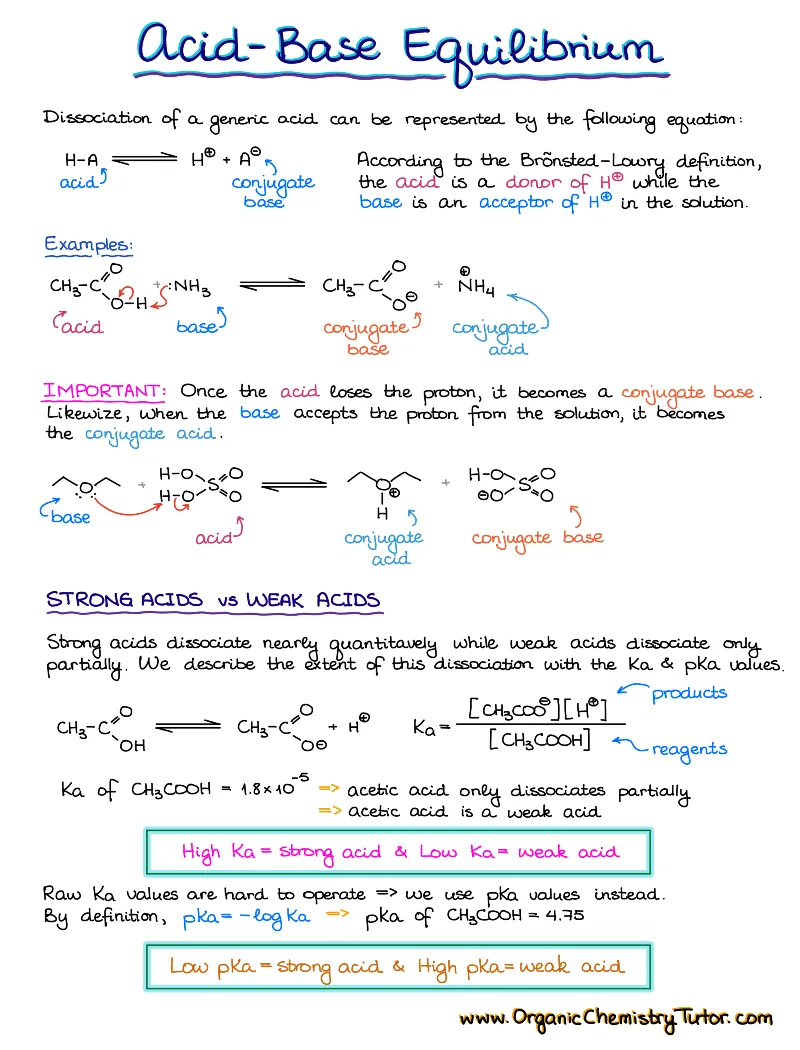
Acid-Base Chemistry
Acid-base chemistry is one of those topics that surfaces in almost every topic in organic chemistry, so having a good acid-base reference and review notes is a must!
What’s Included:
- Bronsted-Lowry and Lewis acids and bases
- Acid-Base equilibrium and what influences it
- How to predict the state of the acid-base equilibrium
- Typical and tricky acid-base exam questions with a complete explanation of how to approach those
- Handy 1-page pKa table cheat sheet

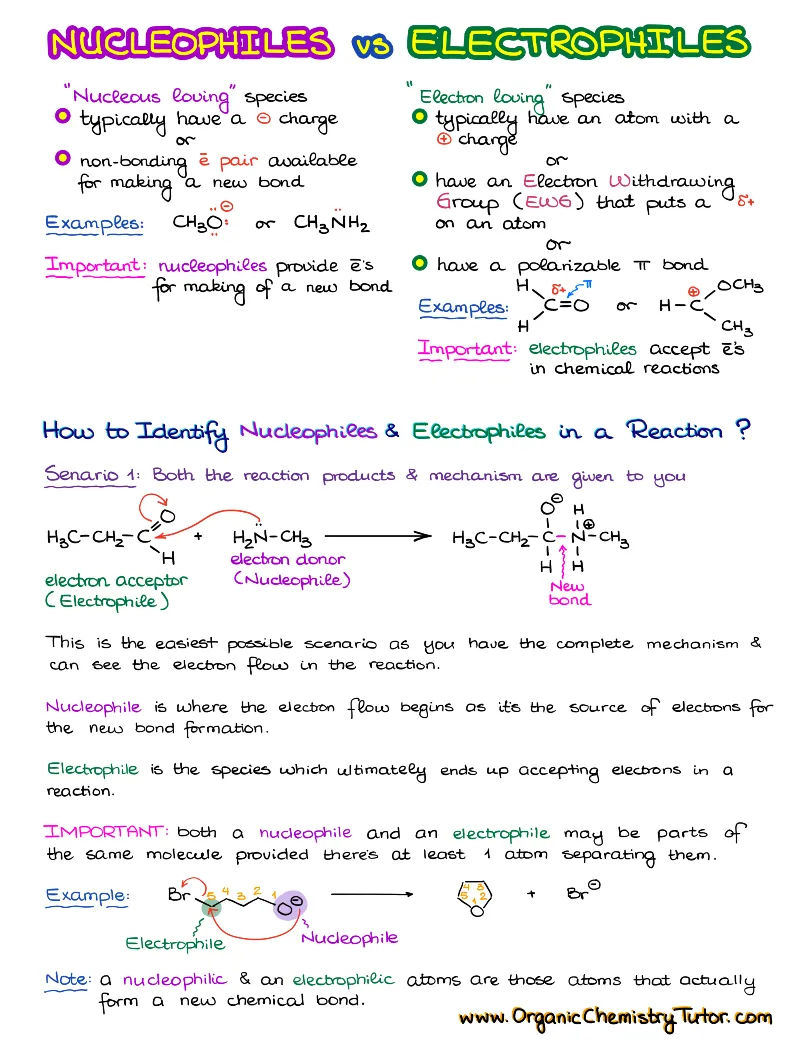
Substitution and Elimination Reactions
Substitutions (SN1 and SN2) and Eliminations (E1 and E2) are the four most common mechanisms in organic chemistry. You are going to need those for your course. You are likely going to struggle with those reactions without some extra guidance. I’m not gonna sugarcoat if for you. This topic is hard.
What’s Included:
- Unimolecular Substitution SN1
- Unimolecular Elimination E1
- Bimolecular Substitution SN2
- Intramolecular Substitution Reactions (Cyclizations)
- Bimolecular Elimination E2
- Factors Influencing the Rate and Stereochemistry of the Substitution and Elimination Reactions
- A Predictive Model that will help you decide which mechanism you’re looking at

Spectroscopy (IR, NMR, Mass-Spec)
Spectroscopy is taught in every sophomore organic chemistry class. So, yep, spectroscopy is one of those absolute must-know topics. While some professors include spectroscopy in the first semester organic chemistry, and others in the second, you’ll come across is anyways! You’ll also need to know spectroscopy for the MCAT, PCAT, DAT, etc. And experience shows that there are only two things that will help you ace this topic: constant practice and a solid set of spectroscopy cheat sheets and reference tables.
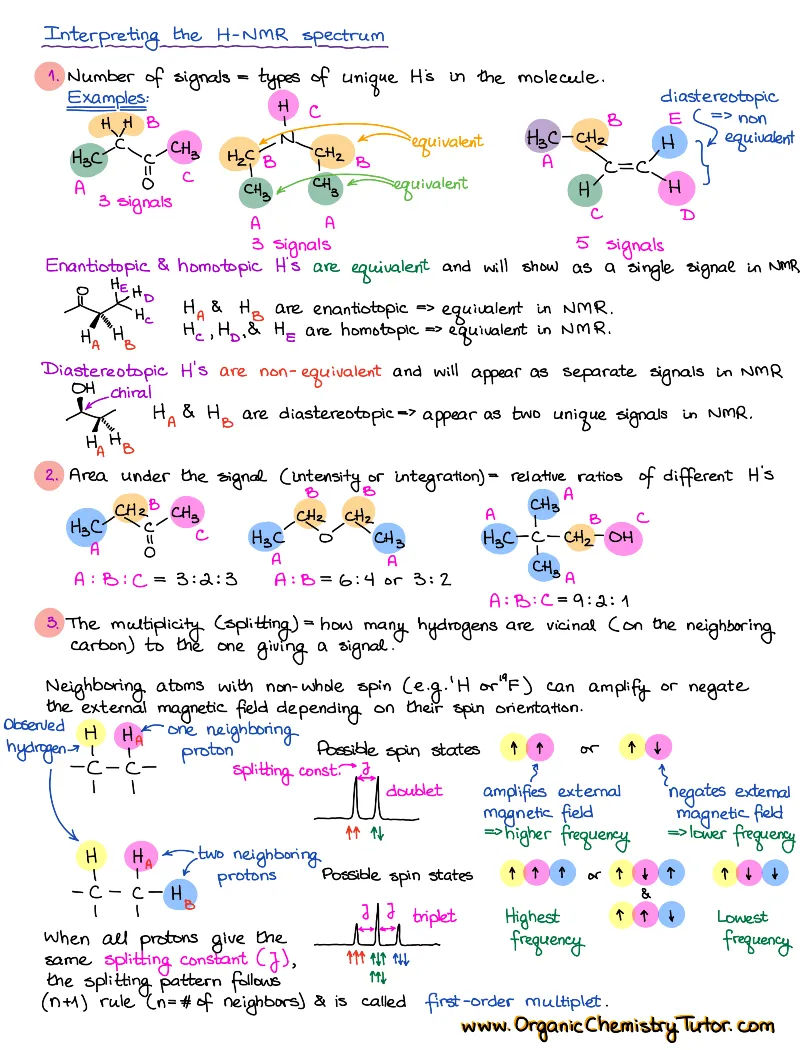
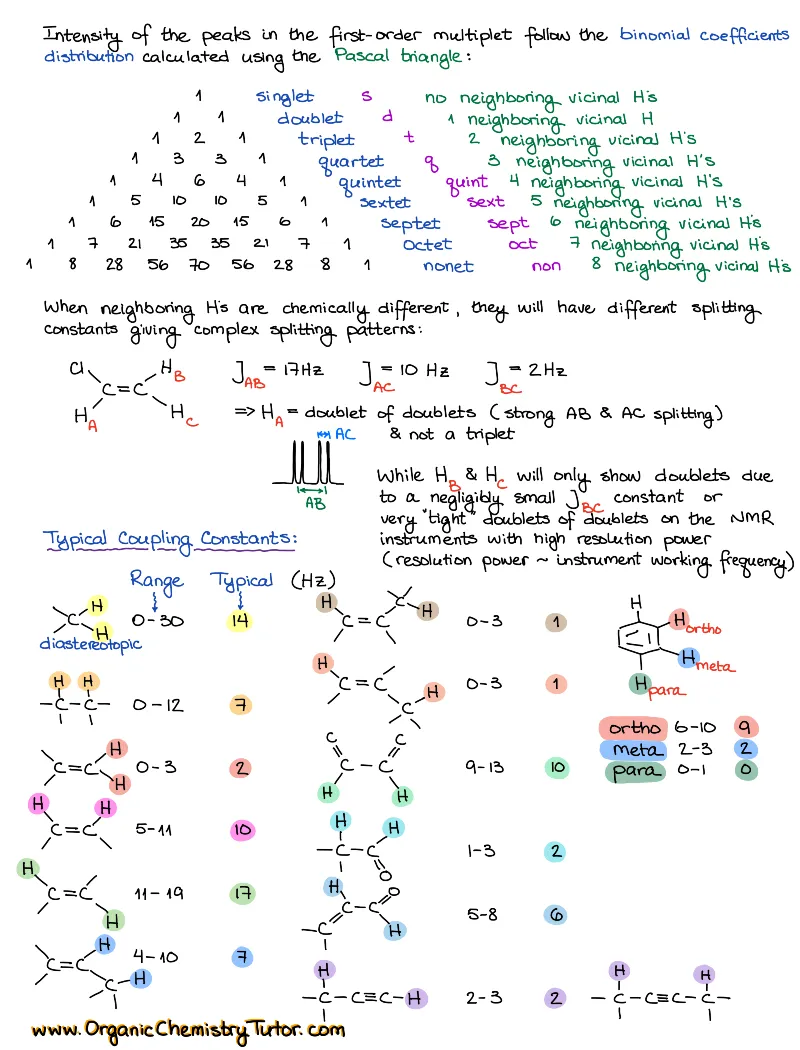
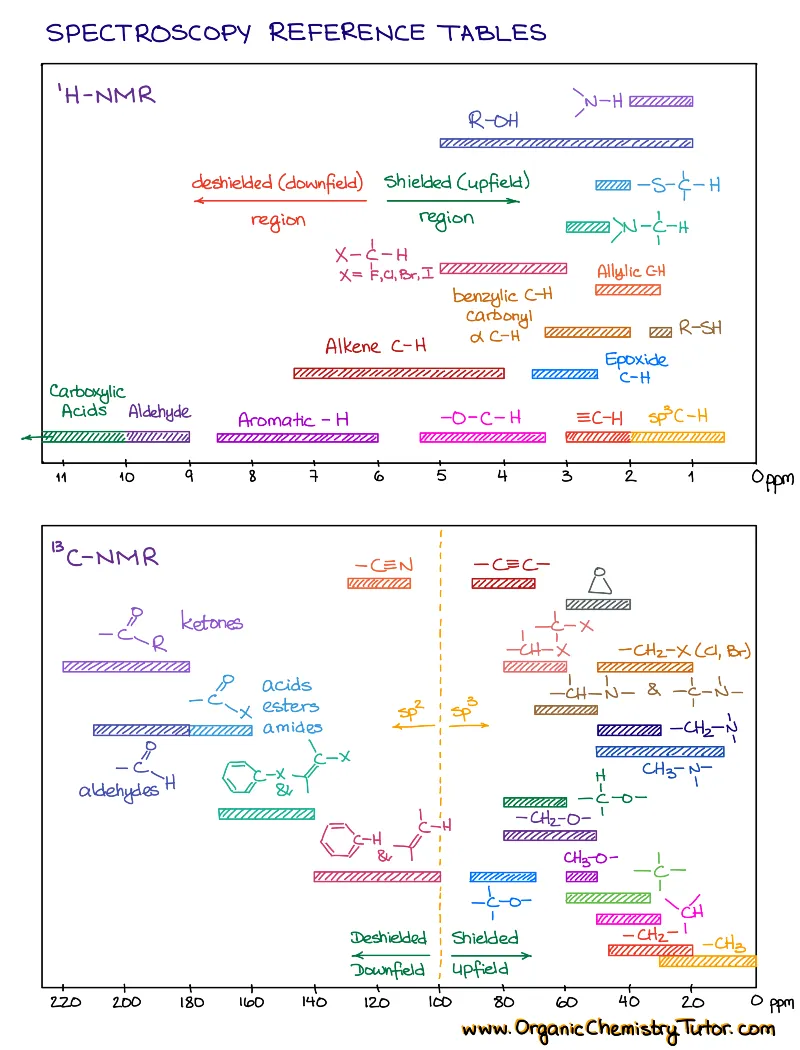
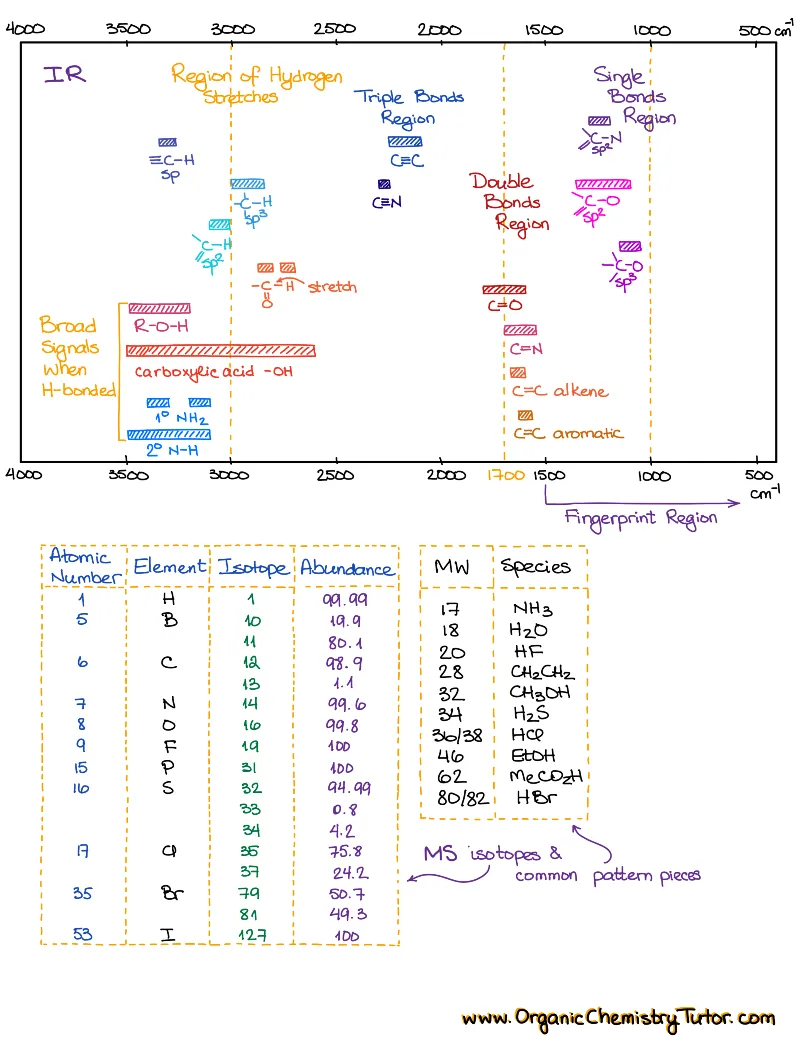
How to study spectroscopy topics?
There are 4 major topics in the spectroscopy “module” covered in a typical organic chemistry course:
- Infrared spectroscopy (IR)
- Proton Nuclear Magnetic Resonance (HNMR)
- Carbon Nuclear Magnetic Resonance (CNMR)
- Mass Spectrometry (Mass-Spec or MS)
If you want to be successful in spectroscopy, you’ll need the following:
- a firm knowledge of the underlying theory
- chemical shifts for NMR and IR stretches
- typical fragmentation patterns
So, many students try (and fail) to just memorize a bunch of meaningless numbers from their spectroscopy reference tables and hope they’ll be able to use that on the exam. What you wanna do instead is practice, then practice more, and when you’re done practicing, do a few more practice problems. I’m not joking. Spectroscopy theory, for the most part, is very simple. The challenge is in the application of the theory. Spectroscopy is, essentially, a puzzle. There’s a growing body of research on teaching spectroscopy to undergraduate students (check this, this, this, and this for examples). And guess what all teachers and researchers say about spectroscopy:
- it is a puzzle
- it requires a solid set of reference or cheat sheets
Many instructors give some sort of spectroscopy sheets to their class. And let’s be frank here, those sheets are usually pretty bad! You don’t need a laundry list of every possible chemical shift or IR stretch! I kid you not, I once had a student who’s instructor gave them 17-page long table with NMR chemical shifts!
How are these spectroscopy cheat sheets better?
I wrote this set with a student in mind and how students use spectroscopy cheat sheets. I color-coded them so they are very easy to navigate. Each topic only contains the essential chemical shifts and IR stretches that you need to solve your spectroscopy puzzles and no unnecessary fluff! I also give you the shifts and stretches in the table and visual forms to help you relate with where to find those signals in the spectrum. Finally, each topic has a brief and carefully written theory portion to give you just what you need to ace your conceptual test questions.
How to use spectroscopy reference tables
When you’re working on solving the spectrum, have your tables ready. If you have the IR, then have the IR table handy, if you have an NMR spectrum, pull up the NMR table.
- Then, go through each signal in NMR and major signals in IR above 1500 cm-1 and list them on a piece of scratch paper.
- Identify the types of bonds and functional groups you have in your molecule based on the IR stretches.
- Do the splitting pattern analysis (see the notes in the HNMR sections for how to do that).
- List out your puzzle pieces and put the molecule together.
- Move to the next problem!
Since spectroscopy requires a lot of practice to build your puzzle-solving skills, you wanna commit yourself to solving at least 2-3 spectroscopy problems daily. Make it into a daily routine. When I was a student myself, I used to take a 45-min train trip to and from school every day. My train ride was the time to work on the spectroscopy problems. So, while my peers were chatting, reading magazines, or window-gazing, I’d pull out my spectroscopy reference tables, a few spectra printouts, and work on those. By the time the finals rolled in, I’ve solved staggering 700+ spectroscopy problems! Needless to say, the spectroscopy questions on the exam took me no time or effort to solve.
Alkenes, Alkynes, Aromatic Compounds, Alcohols, Aldehydes & Ketones, Carboxylic Acids and Derivatives, Enols and Enolates, Amines…
Detailed notes on chemistry of Every! Single! Functional! Group! you’re going to see in your course. We’re talking about dozens of reactions with detailed step-by-step descriptions of the mechanisms and important things you must know for the test. If I add an entry for each, this page will turn into an endless canvas you’ll be scrolling forever 😆