Reduction of Alkynes
Alkynes, with their distinct triple bonds, are versatile reactants in the expansive world of organic chemistry. Their reduction opens up a myriad of hydrocarbon possibilities.
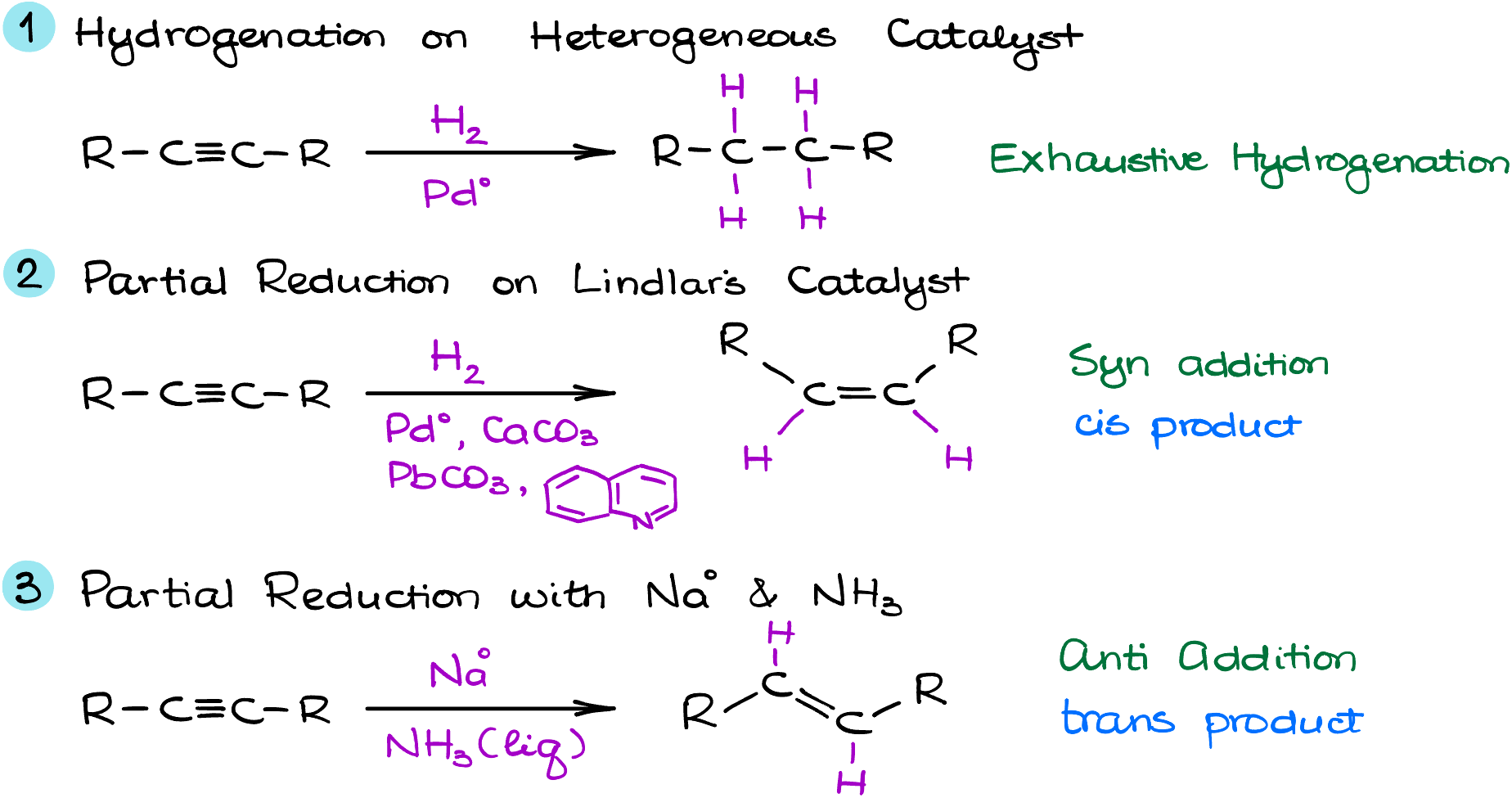
Dive deep into the realm of alkyne reductions, and you’ll find three hallmark techniques, each weaving its unique narrative:
Exhaustive Hydrogenation: Picture an alkyne, a rush of hydrogen, and the shimmering surface of palladium or a similar catalyst. The result? A transformation to alkanes, where the triple bond disappears, giving way to a more saturated compound.
Lindlar’s Catalyst Making the Cis-Alkenes: The magic of Lindlar’s catalyst lies in its ability to partially reduce alkynes. This selective catalyst stops at the alkenes stage, particularly yielding cis-alkenes. It’s a performance where the molecules align side-by-side, embodying harmony.
Alkali Metals in Liquid Ammonia Make Trans-Alkenes: Alkali metals in liquid ammonia are an amazing reducing agent. This method also offers a partial reduction but culminates in the formation of trans-alkenes. Here, molecules stand across from each other, giving a nod to their alkyne origins while embracing a new configuration.
Exhaustive Hydrogenation of Alkynes: The Journey to Alkanes
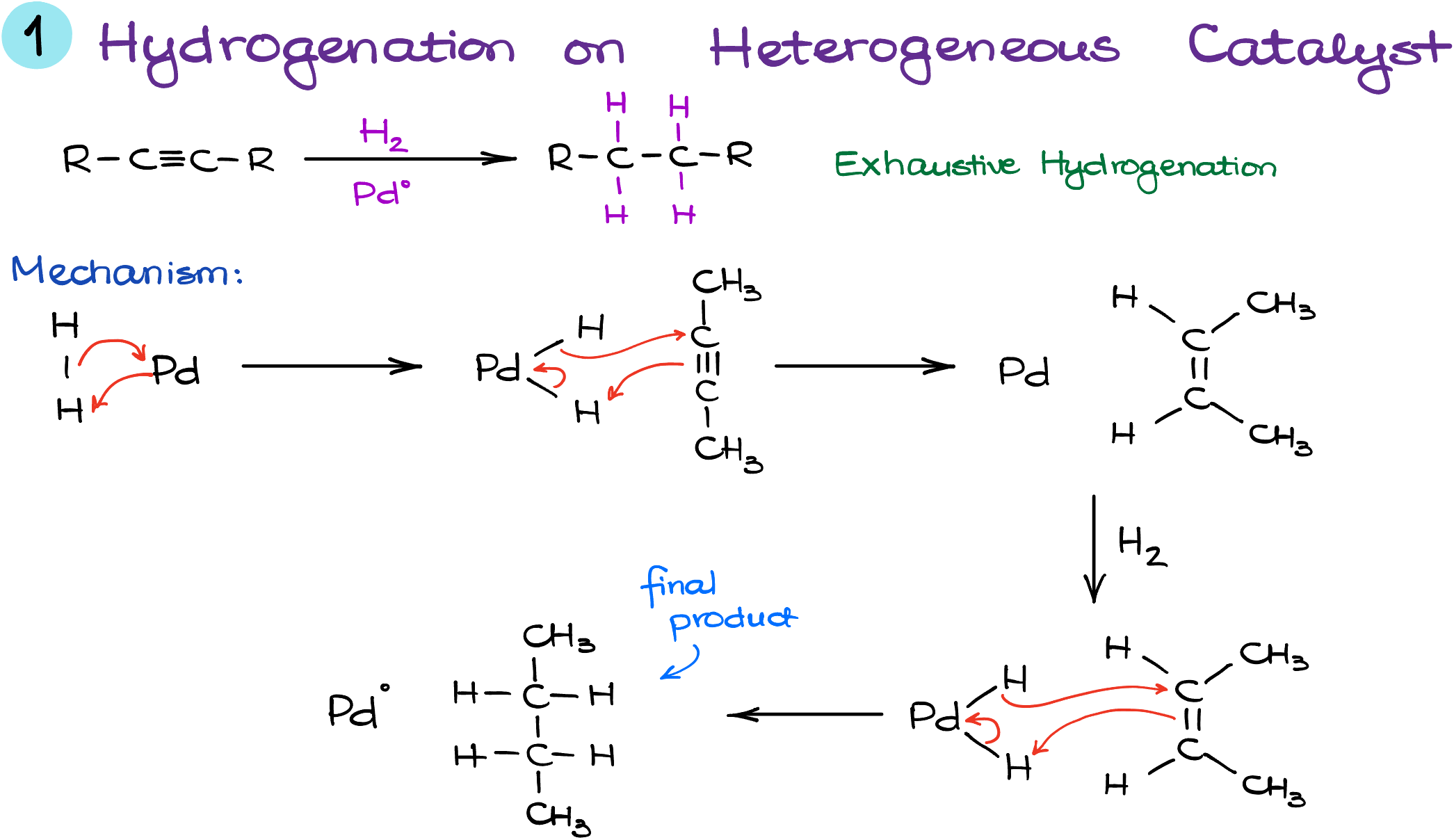
Kickstarting our alkyne reduction journey, we delve into the exhaustive hydrogenation of alkynes—a procedure that transforms the characteristic triple bond into a more docile single bond between two carbon atoms. Let’s break down the process step-by-step:
- Setting the Scene: While there isn’t a quintessential “classic” mechanism for this reaction, we can sketch an illustrative picture of what might be happening on a molecular scale.
- The Role of Hydrogen: Picture hydrogen as an eager actor waiting in the wings, ready to be absorbed by the metal surface. Visualize each hydrogen atom forming a bond with the metal catalyst, like two partners locking arms before a dance.
- Entrance of the Alkyne: As the main character, our alkyne molecule approaches, ready to pick up the hydrogens. Interestingly, it takes both hydrogens simultaneously! This choreography leads to the formation of a cis-alkene, highlighting the stereospecific nature of this reaction and its adherence to the syn addition pathway.
- The Encore: But wait, the dance isn’t over. The newly formed alkene steps up once again, eager for another spin on the floor. It picks up another pair of hydrogens from the metal’s surface, deepening its bond.
- The Grand Finale: The result? A molecule that has shed its alkyne identity, embracing the form of an alkane. In the grand tapestry of organic chemistry, this reaction acts as the go-to method if one wishes to erase that distinctive triple bond. Sure, alkynes have a rich portfolio of reactions, but sometimes, simplicity calls.
- A Familiar Cast: It’s worth noting that the supporting cast for this transformation isn’t new. Catalysts such as Pt, Pd, and Pd-C, which you might recognize from alkene reductions, are equally effective here.
Now that we’ve journeyed from an alkyne to an alkane, it’s time to embrace the more intricate pathways that alkynes have to offer. Buckle up; things are about to get even more captivating!
Lindlar’s Catalyst: The Art of Controlled Hydrogenation
While exhaustive hydrogenation of alkynes fully eliminates the triple bond, sometimes we yearn for a half-step. Enter the Lindlar’s catalyst—a key player that knows just when to pause.
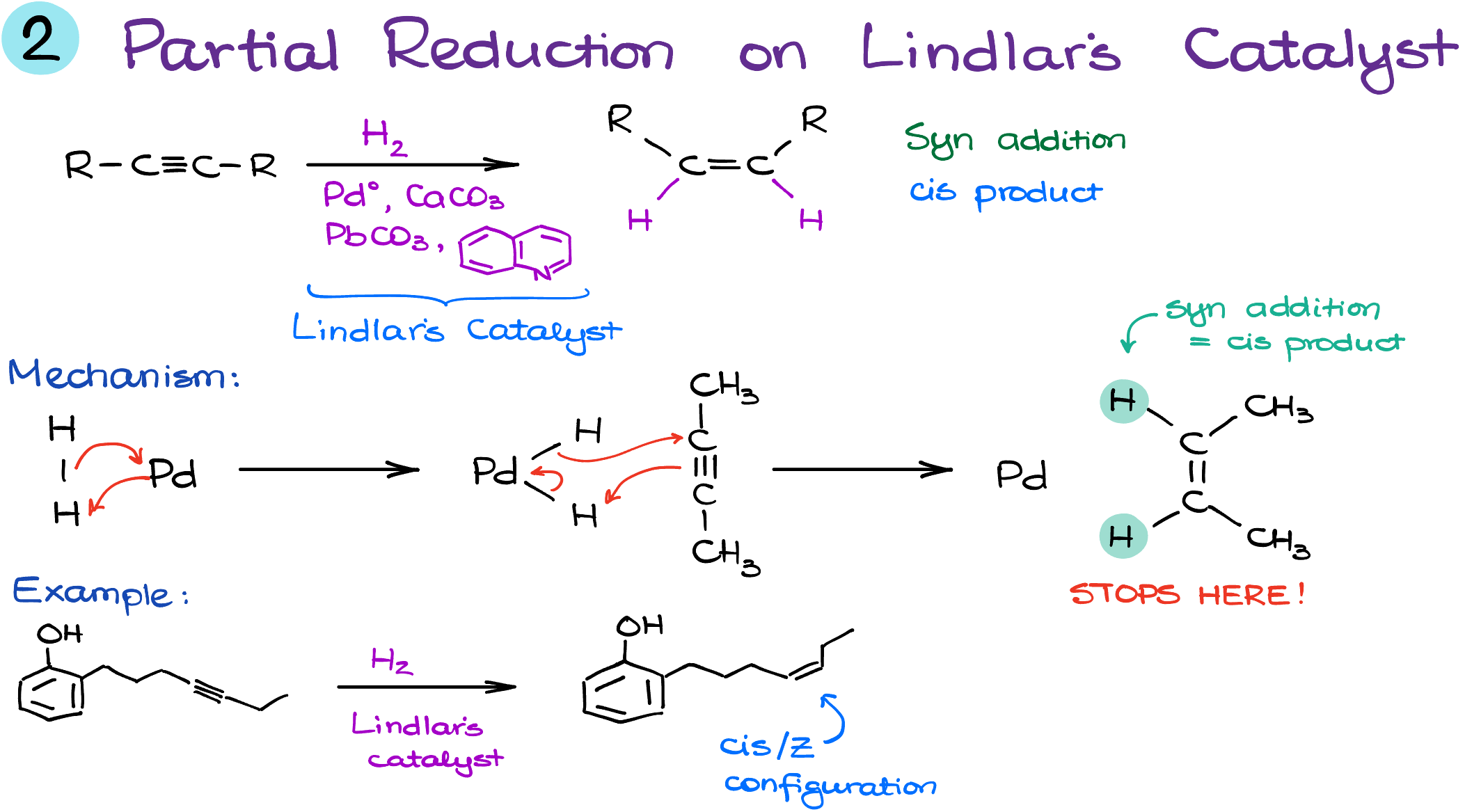
The Need for Halfway: You might wonder, why not go the full distance and transform the triple bond into a single one? It’s because sometimes, moderation is the key. And for such occasions, the Lindlar’s catalyst is our ideal companion.
Meet the Catalyst: Imagine palladium, finely suspended on a bed of calcium carbonate, carefully moderated by lead and quinoline. This composition ensures that our reaction halts precisely at the alkene stage.
Mechanistic Insights: Think of this process as a dance that stops at the first chorus. Mechanistically, it mirrors the initial step of the exhaustive hydrogenation but knows when to bow out. This selective reduction forms a double bond and resists going further.
A Revolution in Synthesis: Cast your mind back to the 1950s, a time of rock ‘n’ roll and groundbreaking chemistry. Lindlar’s discovery was monumental, offering scientists a precise tool to partially reduce alkynes. But there’s a catch! This reaction ensures a syn addition, resulting in a cis alkene.
Partial Reduction of Alkynes with Alkali Metals: The Dance of Electrons and Anions
As we journey further into the realm of alkynes, we find yet another fascinating reaction. This time, we’re diving into the world of alkali metals in liquid ammonia. And trust me, it’s a show worth watching!
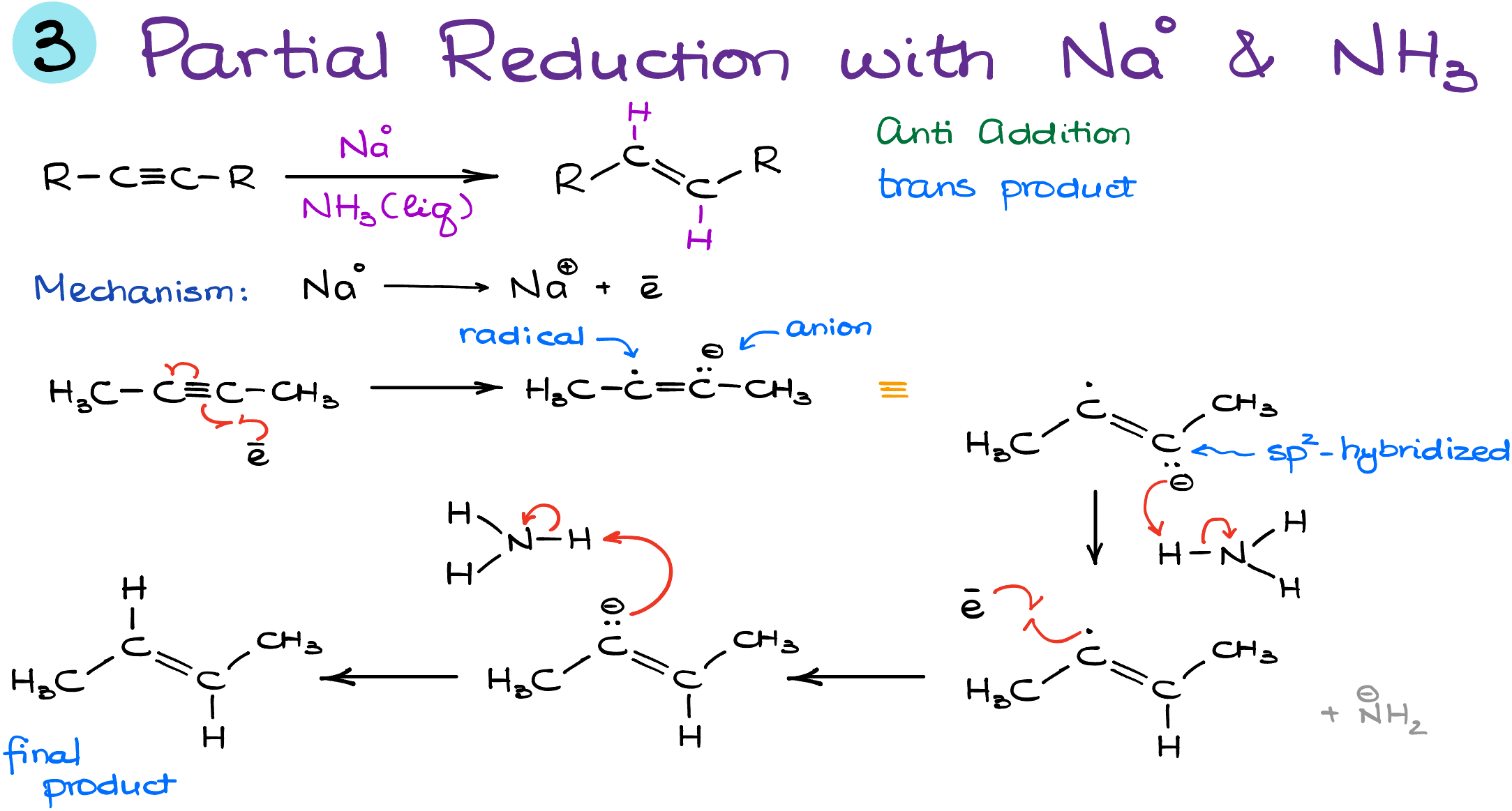
- The Alkali Adventure: Sodium, lithium, or if you’re feeling audacious, potassium. The choice is yours. Just a tidbit for the curious souls: solutions of sodium or potassium in ammonia paint the canvas blue. It’s truly a sight to behold!
- Blue Electron Magic: What gives this solution its striking hue? It’s the electrons from sodium, solvated and swimming freely. Quite an unconventional scenario, right?
- Enter the Anion-Radical: As our solvated electron collides with the alkyne’s pi-bond, a unique entity emerges—an anion-radical. Visualize one carbon holding a radical, while its sibling proudly wears an electron pair as a negative charge.
- Geometry Takes a Turn: Our anion-radical isn’t as straight-laced as our initial alkyne. Undergoing sp2-hybridization, it bends into a familiar zig-zag posture.
- Proton Shuffle: Ammonia steps up to gift our molecule with protons. Meanwhile, the dance floor also sees the birth of an amide anion. The lingering radical isn’t left behind—it snares another electron.
- The Final Steps: With a fresh electron in tow, another anion emerges, only to snatch a proton from ammonia, crafting our final trans product.
If you’re not dizzy from this electron extravaganza, hats off to you! Now, for some candid advice. While the depths of this mechanism might not always grace your exams, it’s imperative to remember the outcomes: a trans product from alkali metals and a cis product with Lindlar’s catalyst. So, gear up, check with your prof, and be prepared!
Chemical Trickery with Terminal Alkynes: A Tale of Twists and Turns
Dive right into the heart of chemistry, where things are seldom as they first appear. Picture hept-1-yne undergoing our recently discussed alkali metals in ammonia reaction. You’d naturally expect a double bond to form, right?
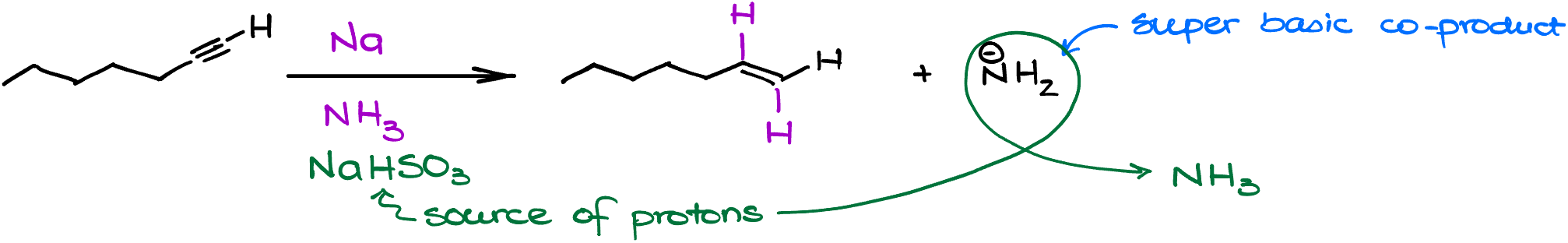
Well, sometimes, even in chemistry, there’s more than meets the eye. Here comes the plot twist: the amide side product we briefly touched upon earlier. These amides aren’t just any kind of basic; they’re on the level of dashing to the coffee shop for the season’s first pumpkin-spice latte. Their first order of business? To target your terminal alkyne and deprotonate it, making the alkyne rather uncooperative.
However, worry not, for every problem in chemistry, there’s a solution waiting to be unraveled. When working with terminal alkynes, always arm yourself with a proton source. This keeps the eager amide neutralized, allowing your alkyne to participate effectively. Conversely, if your heart desires a smoother reaction experience sans the terminal alkyne drama, non-terminal alkynes are your friends. But do be prepared for the lingering aroma of ammonia in your lab. Every experiment has its own signature, after all!
Lindlar’s Catalyst: The Dependable Player in Alkyne Reactions
Switching gears a bit, let’s talk about the reaction with Lindlar’s catalyst. Unlike some of the previous examples we discussed, Lindlar’s catalyst doesn’t bring any peculiar limitations or unexpected outcomes to the table. It’s a reliable partner when working with either internal or terminal triple bonds, as demonstrated in our example.
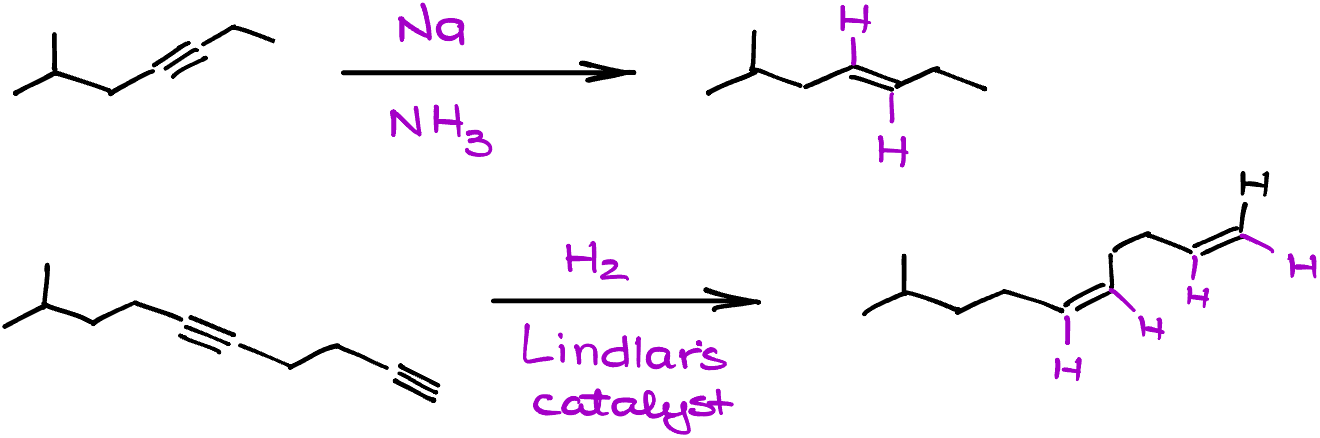
A key thing to note, especially if your molecule boasts multiple triple bonds, is that the reactions we’ve delved into today won’t play favorites. They’re not chemoselective and will engage with all the triple bonds present. But here’s a tidbit to keep in the back of your mind: while Lindlar’s catalyst and sodium in ammonia remain indifferent to any double bonds in your molecule, hydrogen on pure palladium isn’t as discerning. It’s ready to tackle and reduce everything in its path. Always good to remember as you design your experiments!

Let’s put this into perspective with a practical example. In one particular reaction, rest assured, my double bond remains untouched and safe as houses. Yet, in another scenario, all bets are off! Both double and triple bonds find themselves reduced down to single bonds. It’s as if they never existed! And there we have it, a whirlwind tour of alkyne reduction. With these insights, you’re now well-equipped to navigate the fascinating world of organic chemistry reactions. Remember, it’s all in the details!
Concluding Thoughts
When diving into the world of alkyne reductions, there are a few key things to bear in mind. Alkynes can be partially reduced using either the Lindlar’s catalyst or alkali metals in liquid ammonia, with the former yielding a cis product and the latter a trans product. However, be wary of the quirky nature of certain reactions, especially when working with terminal alkynes. While the reaction with sodium in ammonia can deprotonate terminal alkynes, making them unreactive, the Lindlar’s catalyst operates without such hitches. Furthermore, while both the Lindlar’s catalyst and sodium in ammonia preserve existing double bonds, using hydrogen on pure palladium will reduce all multiple bonds, turning both double and triple bonds into singles.
