Conversions of Alcohols into Alkyl Halides
Alcohols are very versatile in their chemistry and transformations. They can undergo dehydration, oxidation, acid-base chemistry, transformations into other functional groups, and other interconversions. In this tutorial, I want to address one of those functional group transformations. Namely, the conversion of alcohols into alkyl halides.
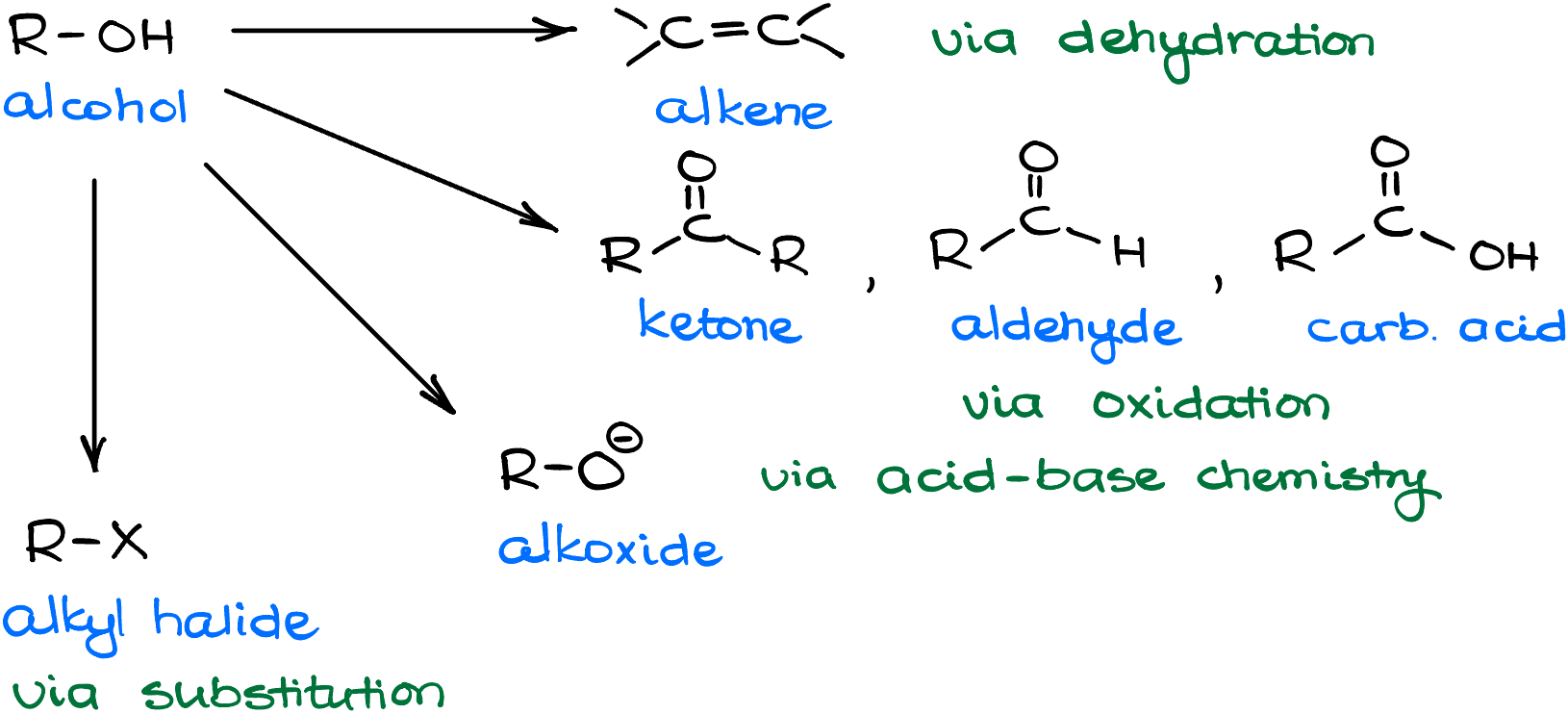
Why do we care about this type of a reaction? Well, we know that the -OH is a bad leaving group. So, sometimes it is a convenient switch if we want to replace an alcohol with something else in a substitution reaction. Or maybe, we want to do an elimination reaction and performing the elimination with the alcohol itself is not feasible. Who knows! The point is, the more functional group transformations we have in our toolbox, the easier it will be for us to accomplish various syntheses. Having options is always a good idea. I doubt you would be happy to go into a diner with only two options on the menu and nothing but a 3-day old coffee to wash it down with. So, same deal we have with organic chemistry—the more transformations we have, the easier it is going to be for us to come up with good ways to accomplish our transformations.
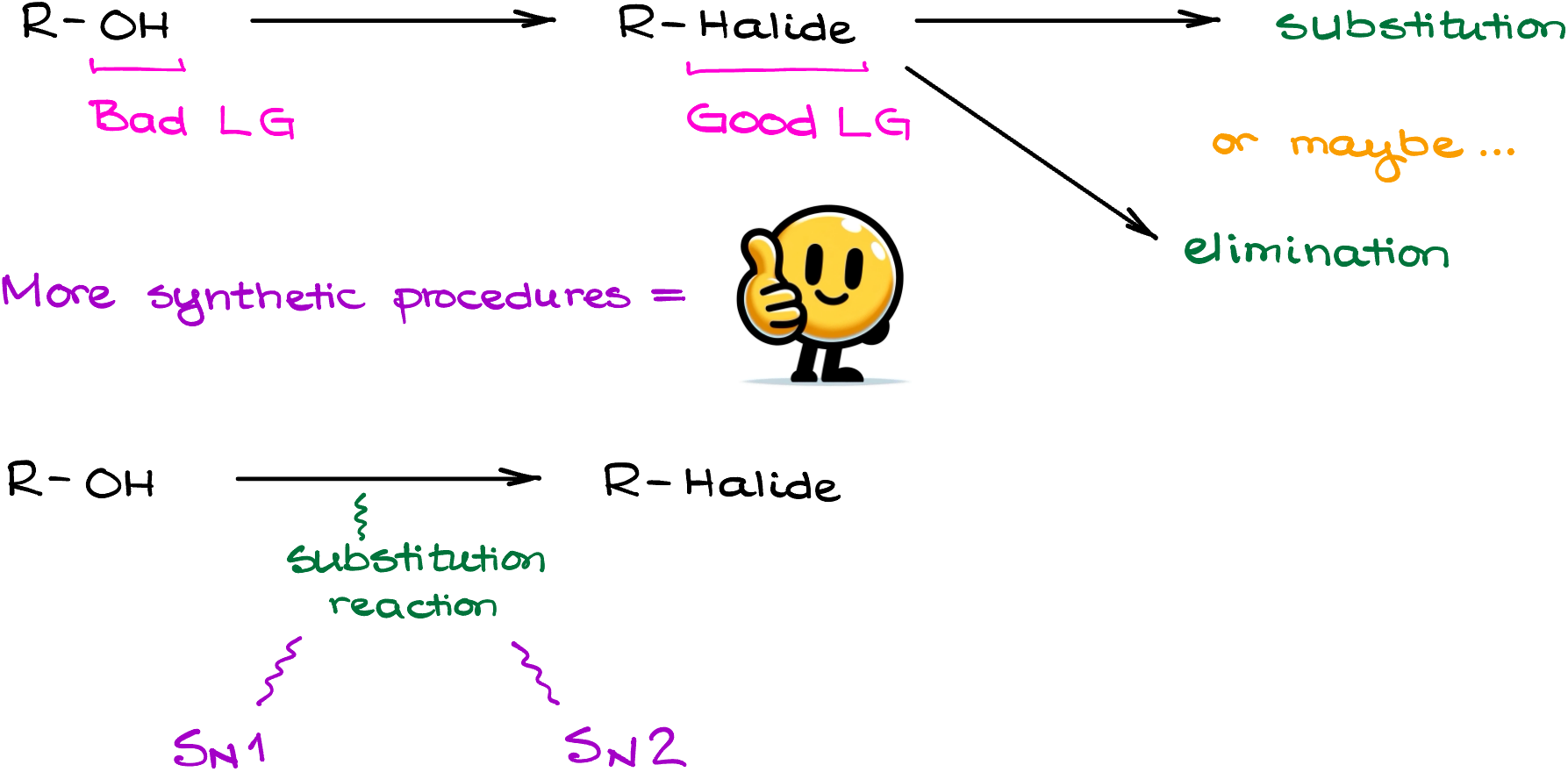
Now, when it comes to the conversion of alcohols into alkyl halides, we’ll be looking at some sort of a substitution reaction to accomplish this transformation. And as substitution reactions go, we have either SN1 or SN2 mechanisms. Let’s start by looking at the SN1 reactions first.
SN1 Conversion of Alcohols into Alkyl Halides
When alcohols react with hydrogen halides like HCl, HBr, or HI, they typically undergo an SN1 reaction yielding the corresponding alkyl halides as products.
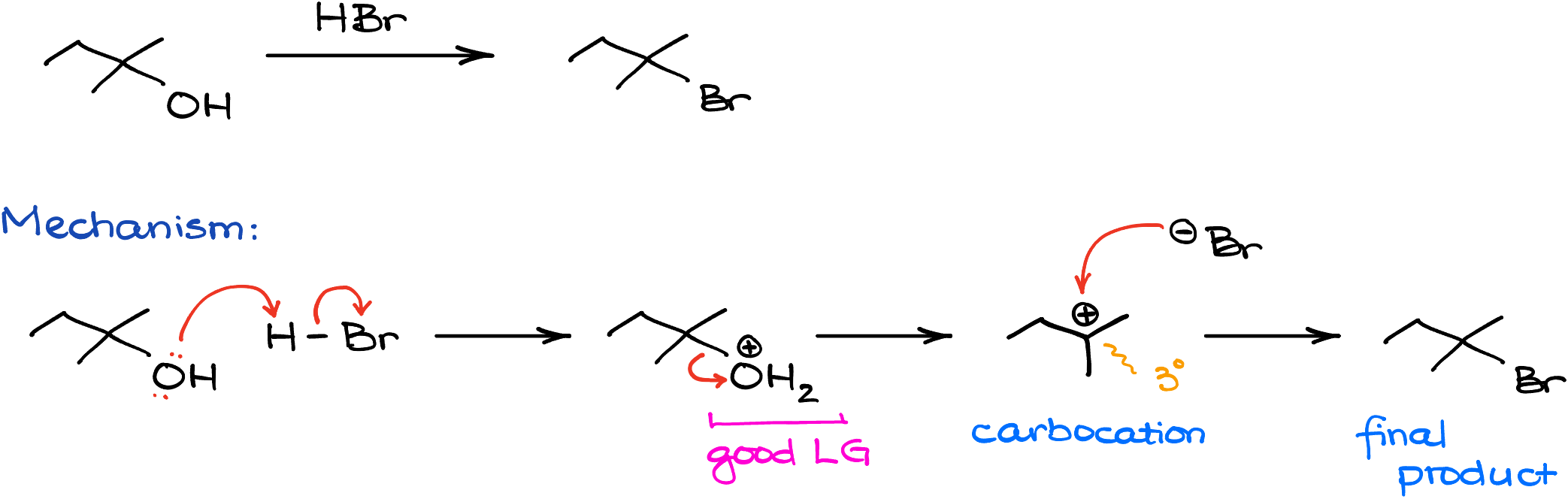
For instance, in this reaction, I’m reacting 2-methylbutane-2-ol with hydrogen bromide. In this case, we’ll make 2-bromo-2-methylbutane as our product. Mechanistically speaking, the reaction will start with the protonation of the -OH group turning it into a good leaving group. Then, once the leaving group dissociates, we’re going to make a corresponding carbocation—a tertiary (3°) carbocation in this case. Next, since we do not expect any carbocation rearrangements, the bromide anion can attack the carbocation, giving us the final product.
Carbocation Rearrangements in SN1 Reactions
As I’ve mentioned a second ago, SN1 reactions form carbocations. And because of that, we need to be very careful with possible carbocation rearrangements. Remember, that if a carbocation rearrangement can give you a more stable carbocation, it will happen before the nucleophilic attack. Which means, that every time we make a carbocation as our intermediate, we always have to check for possible rearrangements. This should become your immediate red flag: I see a carbocation, I check for a possible rearrangement.
So, if I take this secondary alcohol and react it with, say, HI, I’ll end up with the rearranged product. And my iodine will be on a different atom from where -OH group used to be.
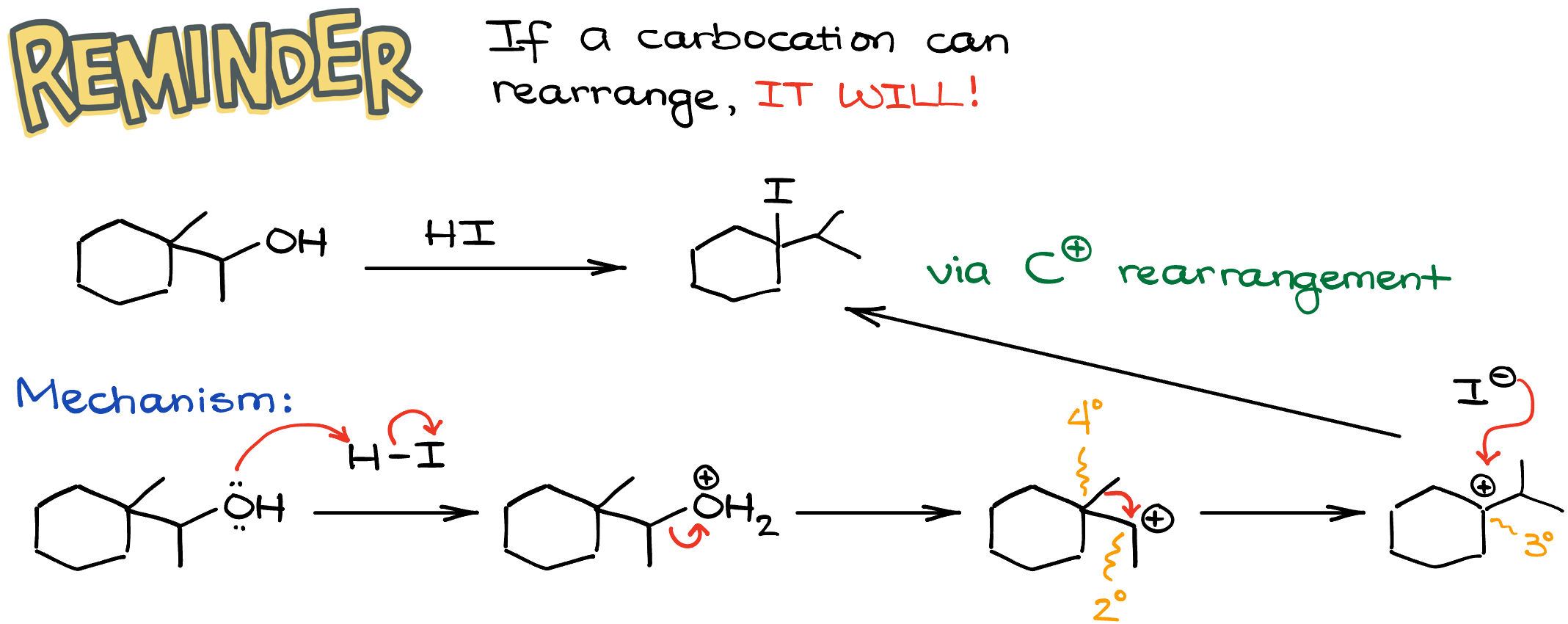
Let’s look at the mechanism here, so we can see exactly how that happened. First, we’ll protonate the -OH group making it into a good leaving group. Then, we’ll have a leaving group dissociation, yielding a carbocation. Now, this carbocation is secondary. And it is right next to a quaternary (4°) carbon. This means that we can perform an alkyl shift and make a more stable carbocation. The methyl shift here makes a new tertiary (3°) carbocation. Now, since my carbocation is as stable as it can possibly be in this case, I’ll proceed with the nucleophilic attack. And once the iodine attacks the carbocation, we’ll end up with the final product. I do have a whole tutorial on the carbocations and carbocation rearrangements. So, if you feel like you may need a refresher, check out this topic.
SN2 Conversion of Alcohols into Alkyl Halides
What are we going to do if we don’t want a carbocation rearrangement? Or, what if I have a primary alcohol that won’t even make a carbocation to begin with making the SN1 pathway quite problematic. Well, in this case we’ll have to use a milder and more controlled SN2 conversion method.
There are many different versions of the SN2-style reactions that convert alcohols into alkyl halides. But despite the variability of methods and reagents, they all boil down to the same simple strategy:
- First, we convert the -OH into a good leaving group. This is done in situ.
- And second, we perform the SN2 attack to get the target halide.
In this tutorial, I’ll go over one of those reagents, which is probably going to be one of the most common ones you’ll be seeing in your class—PBr3, phosphorus tribromide, in pyridine. But you may also encounter phosphorous trichloride, phosphorus pentahalides, thionly chloride, and many other reagents. Ultimately, it’s up to your instructor and the textbook you’re using which of those reagents you’ll cover in your course. The PBr3, however, is a classic and you’ll definitely see that one in your class.
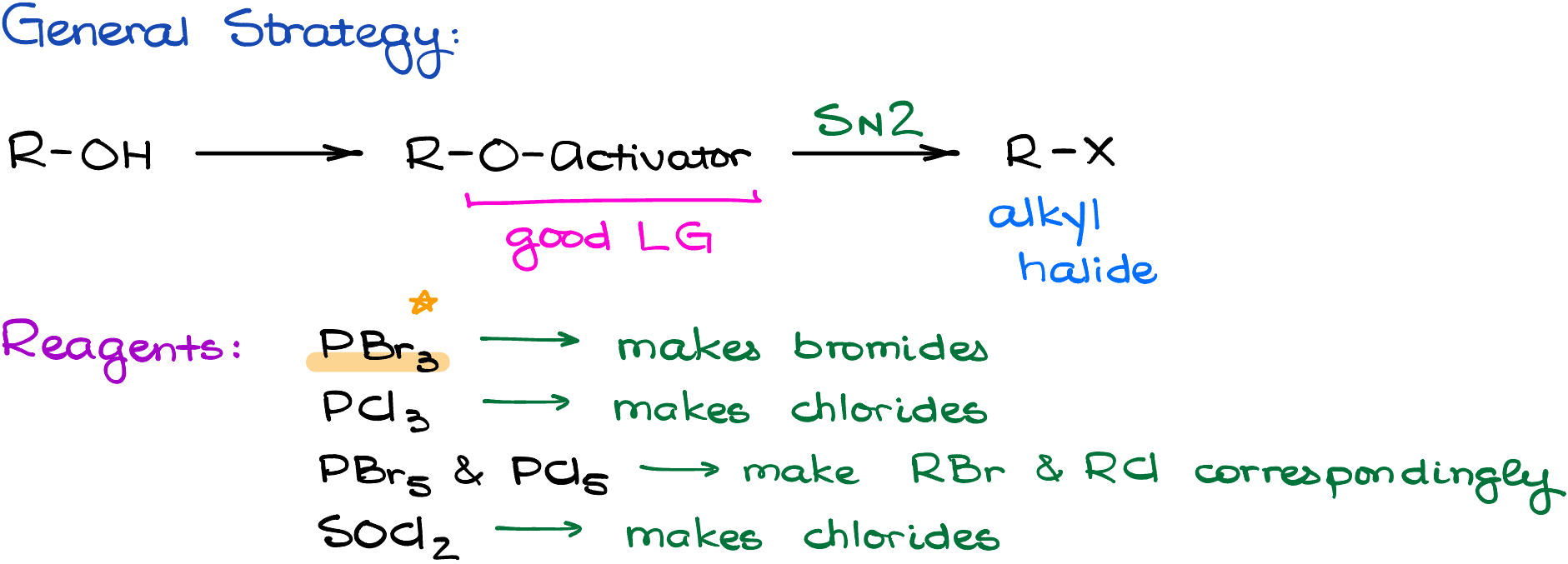
So, if I take the same starting material as in the last example and treat it with PBr3 in pyridine, I’m going to make a corresponding bromide, but now we’re not going to see any carbocation rearrangements.

Mechanistically, this reaction will start from the nucleophilic attack from the alcohol onto the phosphorous tribromide. This gives us the protonated intermediate, which quickly loses the proton to pyridine. Now, we have an excellent leaving group, and the bromide anion can attack our molecule replacing this leaving group. This is a simplification of the actual mechanism. In reality, experimental data suggests that pyridine here is a bit more proactive than just a basic solvent and a way to deprotonate our intermediate. But this is the way we typically show this reaction in a sophomore class, so I’ll stick to this version here.
Also remember, since this is the SN2-style reaction, you’ll have an inversion of stereochemistry if the alcohol is chiral. For instance, if I react (S)-butane-2-ol with PBr3 in pyridine, I’ll get (R)-2-bromobutane as the product. Write down the mechanism of this reaction and make sure you understand how this happened!
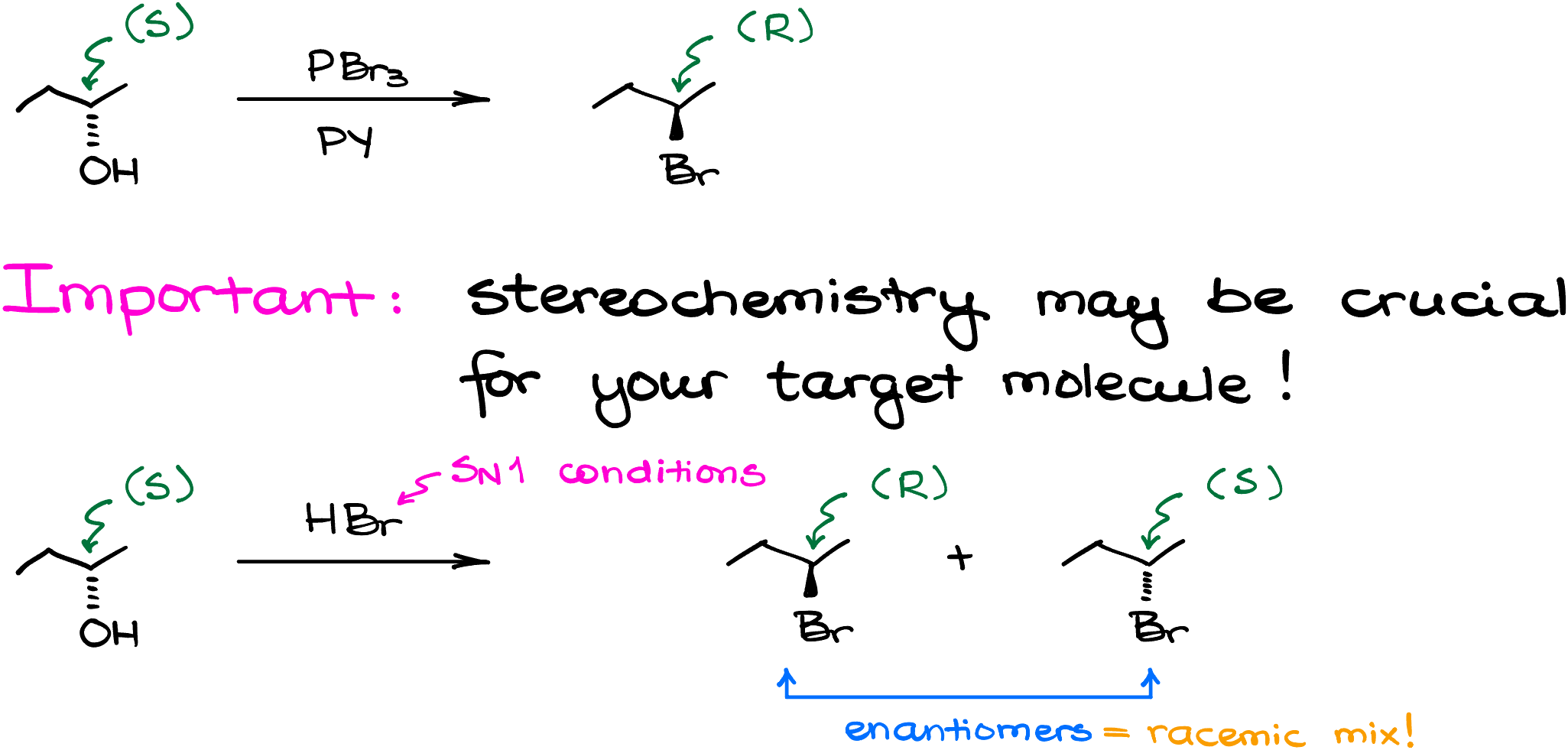
Why do we care so much about the stereochemistry? Well, if you’re planning your synthesis and you need to have a specific stereochemistry of the final product, then knowing whether the reaction is going to retain the configuration, invert the configuration, or scramble it making a racemic mixture will be important to you. If I took same (S)-butane-2-ol and treated it with HBr instead, doing this reaction in the SN1 conditions, I’d end up with the racemic mixture instead of an enantiomerically pure final product. Which may or may not be a problem depending on my needs.
Concluding Thoughts
So, as you can see, you can convert alcohols into alkyl halides using several different methods. If you don’t care about the stereochemistry and carbocation rearrangements are not an issue, feel free to use the SN1 methods. If you need more control over your reactions and stereochemistry is important to you, then the SN2 methods is what you need. At the end of the day, the choice is going to be based on the problem you’re working on. So, like in many tutorials where I’m talking about different reactions, I’ll warn you agains making blanket statements and gravitate towards one or the other method just because you like it. Always analyze the molecule in front of you and make a decision based on your needs.

Now, if you’re still here, first of all, thank you for watching till the end. And as an extra bonus, here’s a challenge question for you: how would you convert (R)-1-phenylprop-2-en-1-ol into (R)-3-bromo-3-phenylprop-1-ene retaining the configuration and keeping the double bond where it is. I’ll give you a hint: you’ll need to do a sequence of two substitution reactions! Write your answers in the comments below!
