Molecular Representations and Bonding in Organic Molecules
The Periodic Table in Organic Chemistry
When exploring the domain of organic chemistry, the vast periodic table’s intricacies might seem overwhelming. Yet, a closer analysis reveals that only a subset of elements are paramount to understanding organic interactions.
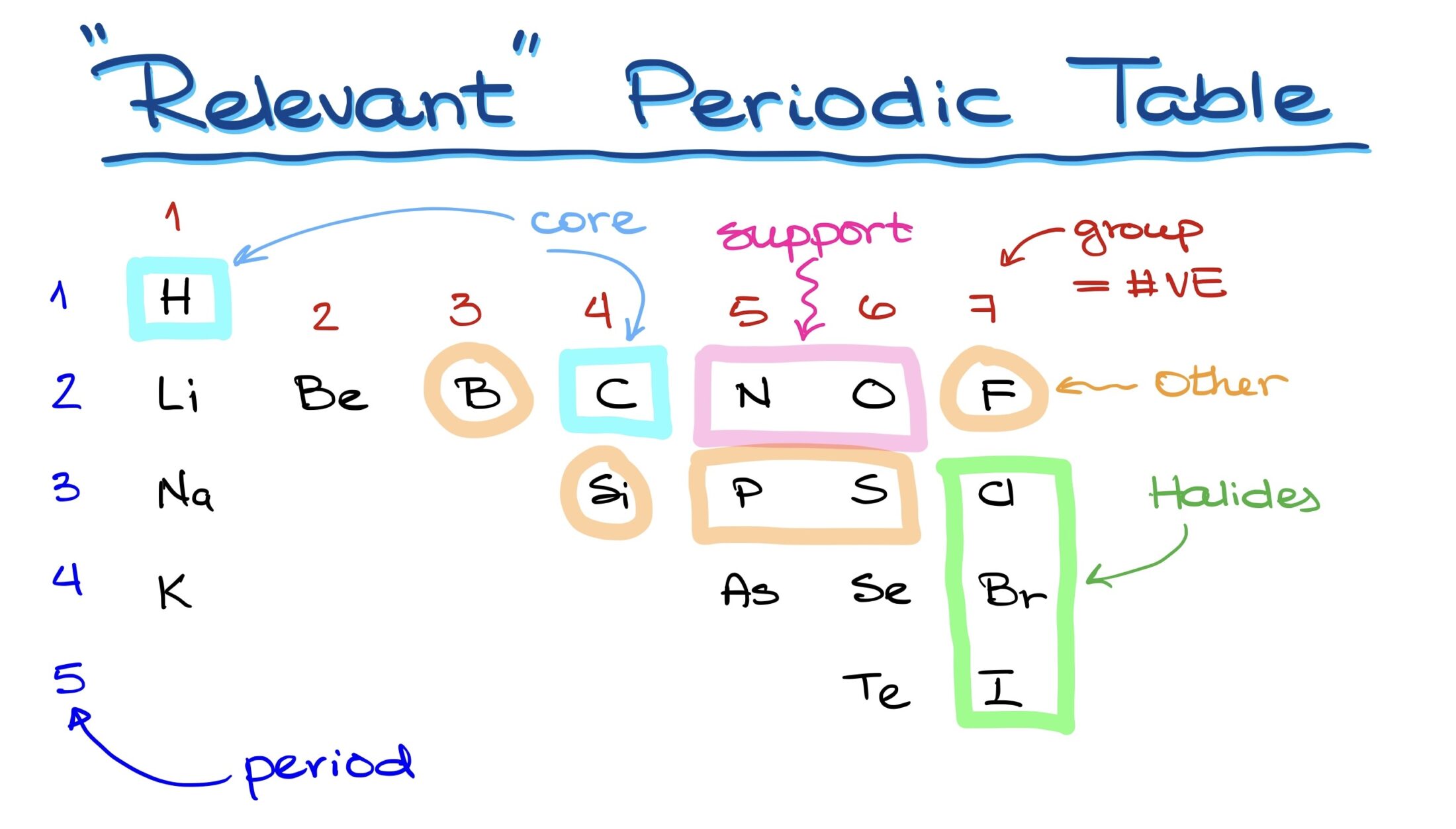
The Core Elements
Unquestionably, carbon is the backbone of organic chemistry. Yet, besides carbon, a selection of core elements also plays a critical role. Within the periodic table, the following are particularly relevant:
- First Period: Hydrogen.
- Second Period: Lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, chlorine, bromine, and iodine.
- Third Period and beyond: Silicon, phosphorus, sulfur, arsenic, selenium, and tellurium beside iodine.
This selection can be deemed as the ‘relevant periodic table of organic chemistry.’ Among these, only lithium and beryllium are metals; the rest are nonmetals commonly encountered in organic molecules.
Key Components in Organic Molecules
While carbon and hydrogen form the essence of organic molecules, nitrogen and oxygen often serve as supporting elements, frequently appearing in various molecular structures. Other occasional participants include halogens like chlorine, bromine, and iodine, along with elements like boron, phosphorus, sulfur, fluorine, and sometimes silicon. However, the latter is often beyond the scope of a foundational organic chemistry course.
Metals in Organic Chemistry
Within the scope of a typical organic chemistry course, metals, especially alkali metals like lithium, sodium, or potassium, are often sidelines. While they don’t entirely vanish from discussions, their primary role is bearing positive charges, acting more as spectators than active participants in chemical reactions. Understanding their profound impact on organic molecules’ behavior usually demands advanced study.
Periodic Table Groupings and their Importance
In the periodic table, ‘groups’ are vertical columns of elements. The group number offers invaluable information regarding the number of valence electrons each element possesses. This insight can further aid in deducing the Lewis structure and, consequently, the element’s chemical properties. Examples of groupings include:
- First Group: Hydrogen.
- Second Group: Contains beryllium.
- Fourth Group: Holds carbon.
- Fifth Group: Nitrogen and phosphorus.
- Sixth Group: Oxygen and sulfur.
- Seventh Group: The halogens.
The eighth group, with noble gases, isn’t critical in organic chemistry discussions due to their inert nature.
Role of Periods in the Periodic Table
Periods in the periodic table refer to the horizontal rows of elements. Although they might seem trivial initially, periods gain significance when discussing charged molecule stability. As we move downwards in the table, elements grow in size, affecting orbital dimensions crucial for electron stability. Yet, for beginners, it’s the valence electrons deduced from an element’s group that remains pivotal.
Predicting Bonds from Valence Electrons
Understanding an element’s valence electrons offers a peek into its bonding capabilities. For instance:
- Hydrogen: With one valence electron, it can form only one bond.
- Boron: With three valence electrons, it forms three bonds.
- Carbon: Having four valence electrons, it can form four bonds.
- Nitrogen: Although it has five electrons, its bonding capacity is three due to paired electrons.
- Oxygen: With six electrons, it can form two bonds.
- Halogens (e.g., Chlorine): Seven electrons allow for one bond.
In graphical representations, bonds are often simplified as lines connecting two atoms, where each line denotes a pair of electrons bonding the elements.
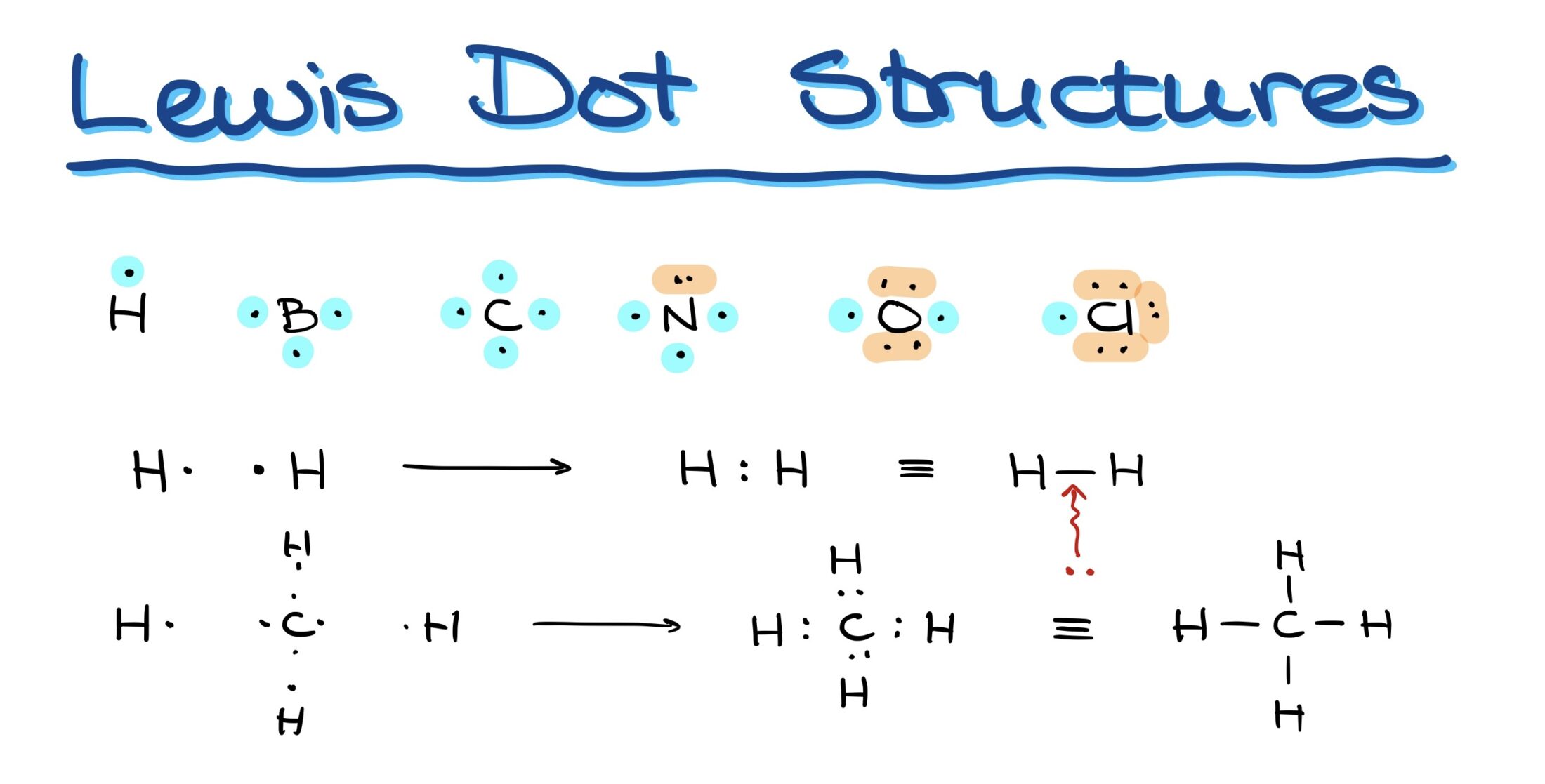
Decoding Molecular Structures with Lewis Structures
Lewis structures act as the foundation for representing organic molecules. These structures use basic building blocks derived from individual atom’s Lewis dot structures. By assembling these blocks, virtually any molecule can be constructed. For example, while C2H6O could represent ethyl alcohol or dimethyl ether, only structural formulas can distinguish between the two.
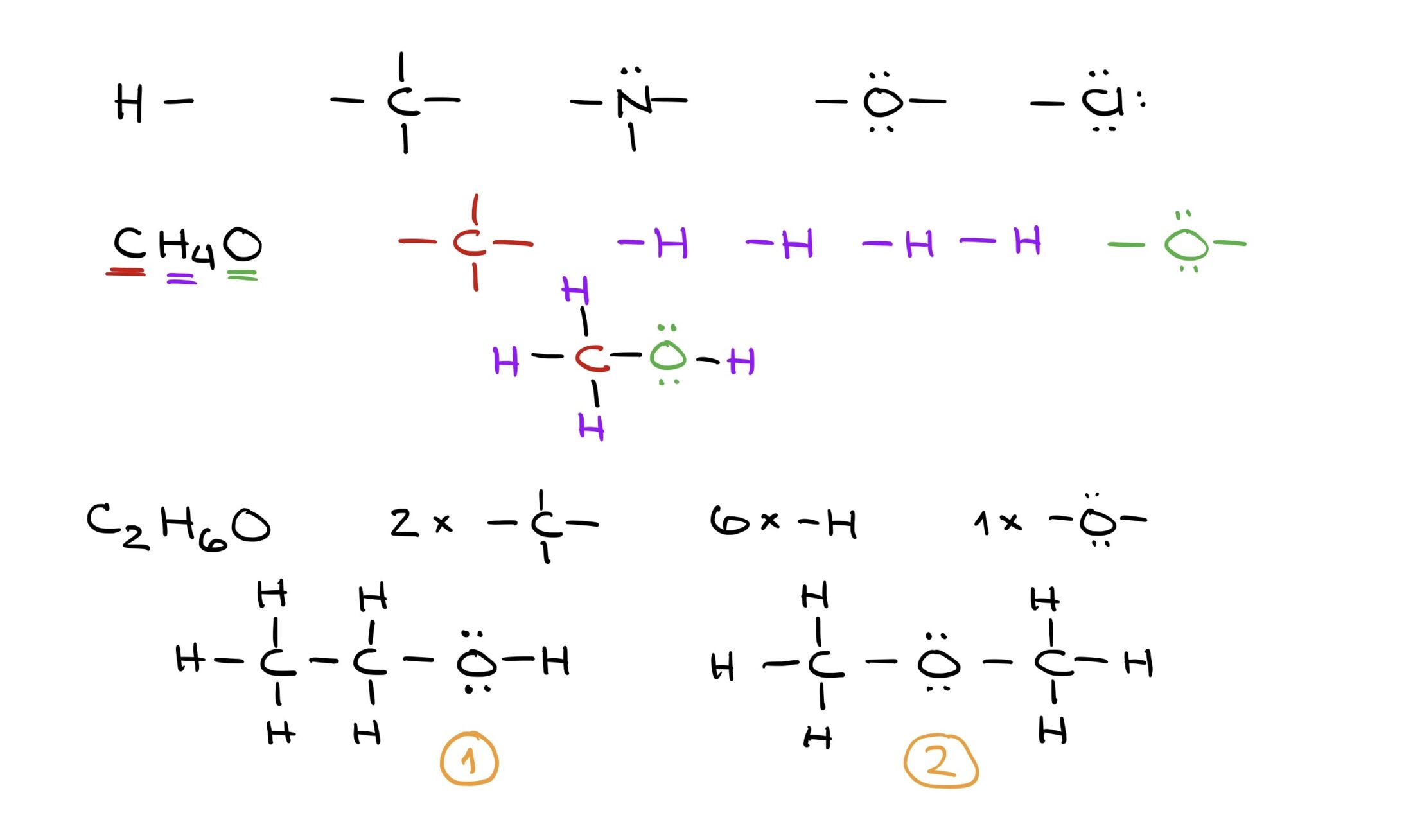
In essence, organic chemistry shifts the focus from simple molecular formulas to detailed structures that capture the molecule’s actual nature.
Crafting Complex Molecules
Building complex molecules demands a clear understanding of basic building blocks and their bonding potentials. Using these foundations, a myriad of structures can be imagined and drawn. For instance, with C3H9N, various configurations can be drafted based on the position of the nitrogen and how the other elements orbit around it.
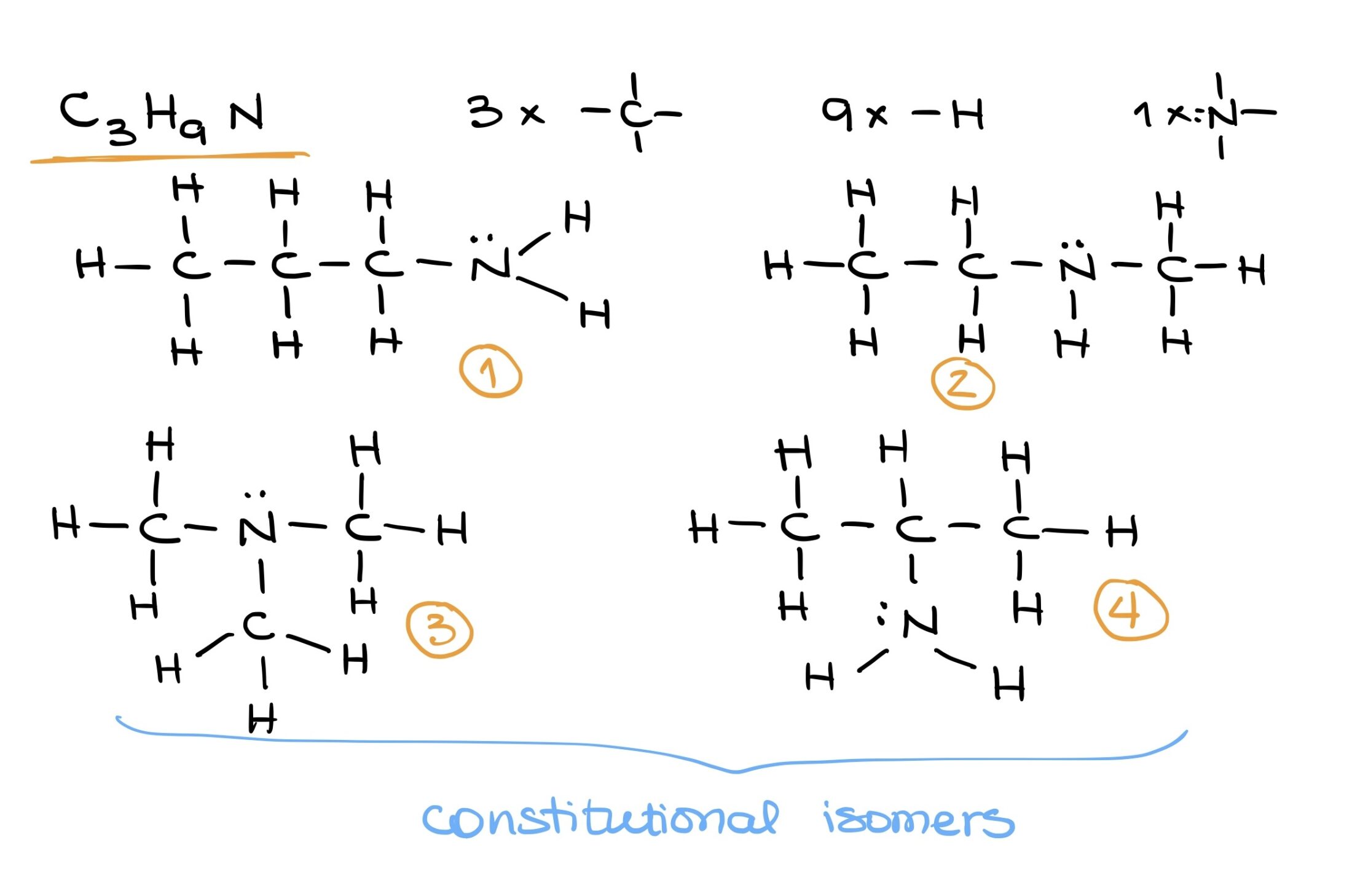
In the following topics we’ll discuss more bonding patterns, Lewis structures, Valence Bond Theory, and other aspects of bonding in organic molecules.
