Cleavage of Ethers with Acids
We’ve already seen how we can make ethers via the Williamson ether synthesis. Now, let’s talk about how we break them! There are several ways how we can cleave our ethers. In this tutorial I’ll focus on the most common one: reaction with hydrogen halides such as HI, HBr, and HCl. And as you might’ve already guessed, it’s going to be either an SN1 or SN2 reaction depending on the structure of our ether.
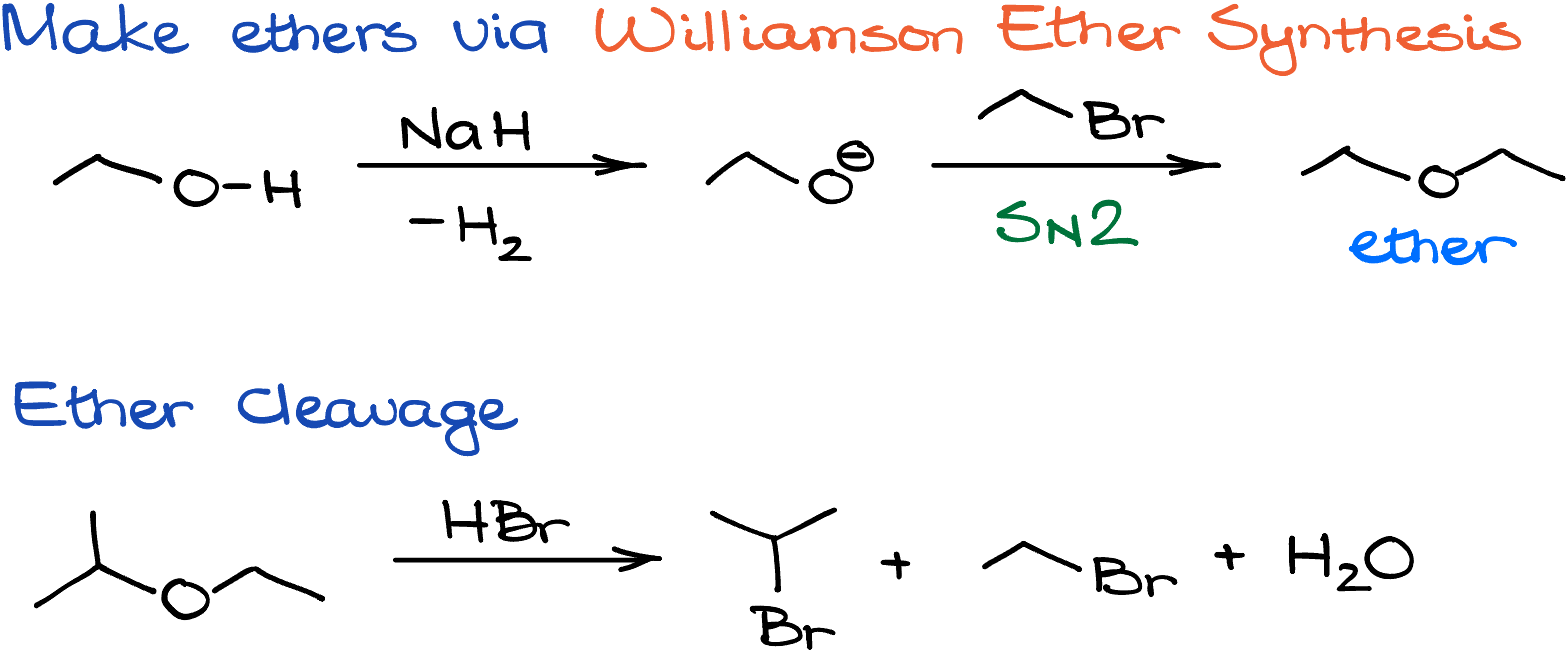
Cleavage of Primary Ethers
To start things off, let’s look at the reaction of diethyl ether with HBr. Since HBr is a pretty strong acid, the first step is going to be the proton transfer, in which we’ll be protonating the oxygen of our ether. This creates a leaving group and we can consider either side of the molecule as a leaving group in this case. Importantly, since this would be a primary leaving group, the SN1 pathway here is impossible despite the protic conditions. This means that we’ll have to attack the carbon directly via the SN2 pathway. This gives us an alcohol and alkyl halide. But since the reaction is typically done in the excess of the HX acid, those are not going to be our end products.
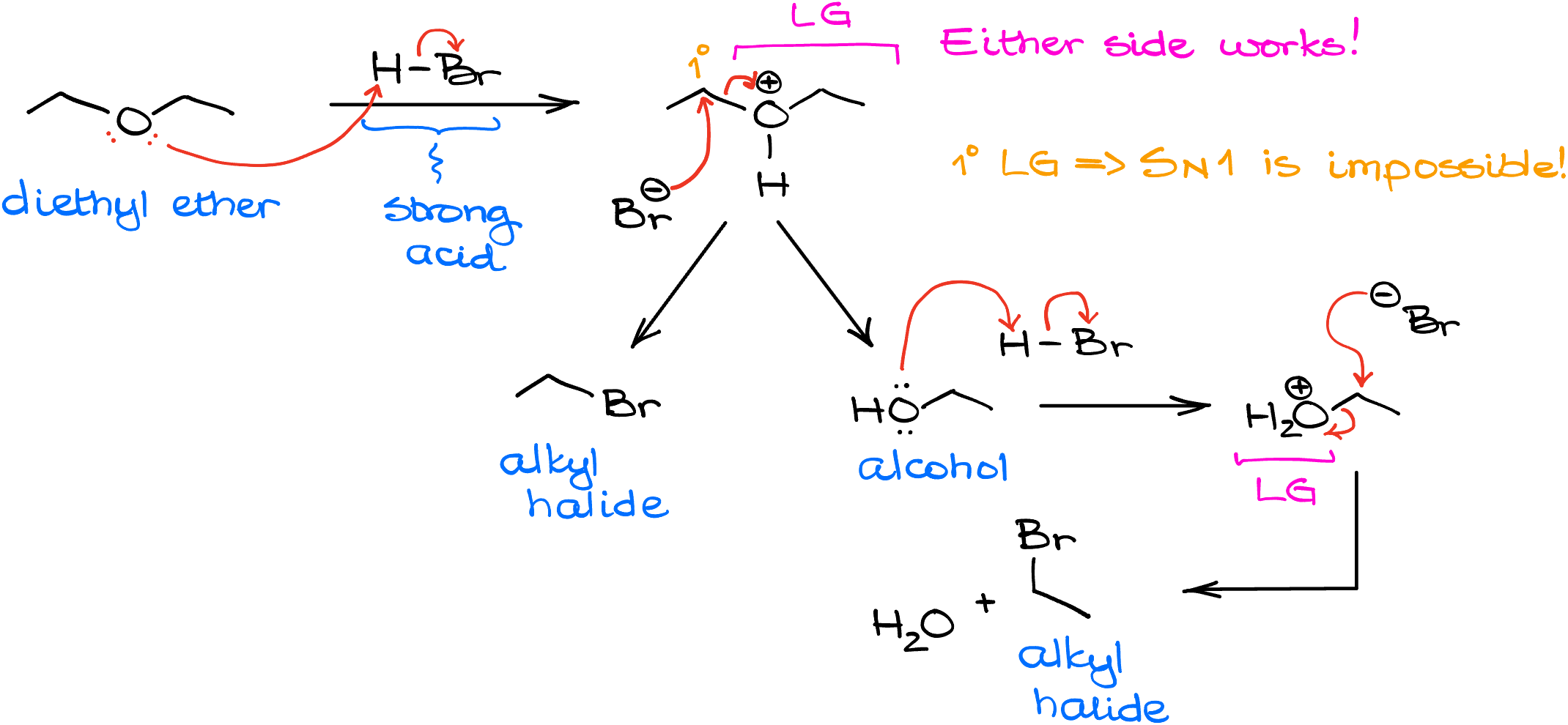
In the next round of this reaction, the HBr attacks the alcohol making H2O leaving group. In this case, just like in the previous round, we cannot form a carbocation as it would be too unstable, so we’ll have another SN2 attack by the halide. As the result, we’ll get another equivalent of the alkyl halide product. In this case, since the molecule is symmetrical, we get bromobutane as our product. However, if molecule is not symmetrical, you’d get two different alkyl halides.
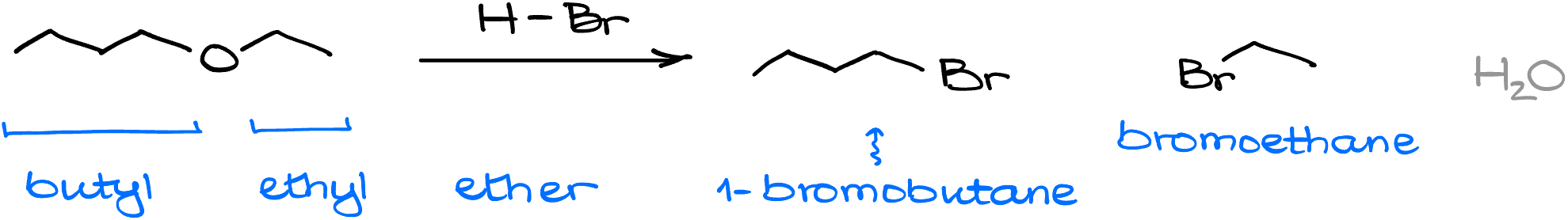
For instance, if I treat this butyl ethyl ether with HBr, one product will be the bromoethane, just like in the previous example. And the other product will be 1-bromobutane. And of course, we’ll have water as our co-product, but as in the case with all inorganic co-products, we don’t really care about it.
Cleavage of Secondary and Tertiary Ethers
If I have tertiary or secondary ethers, I’m going to go through the SN1 mechanism. For instance, if we take this di-tert-butyl ether and treat it with HI, we’ll get two equivalents of tert-butyl iodide, or if you prefer the IUPAC name, 2-iodo-2-methylpropane.
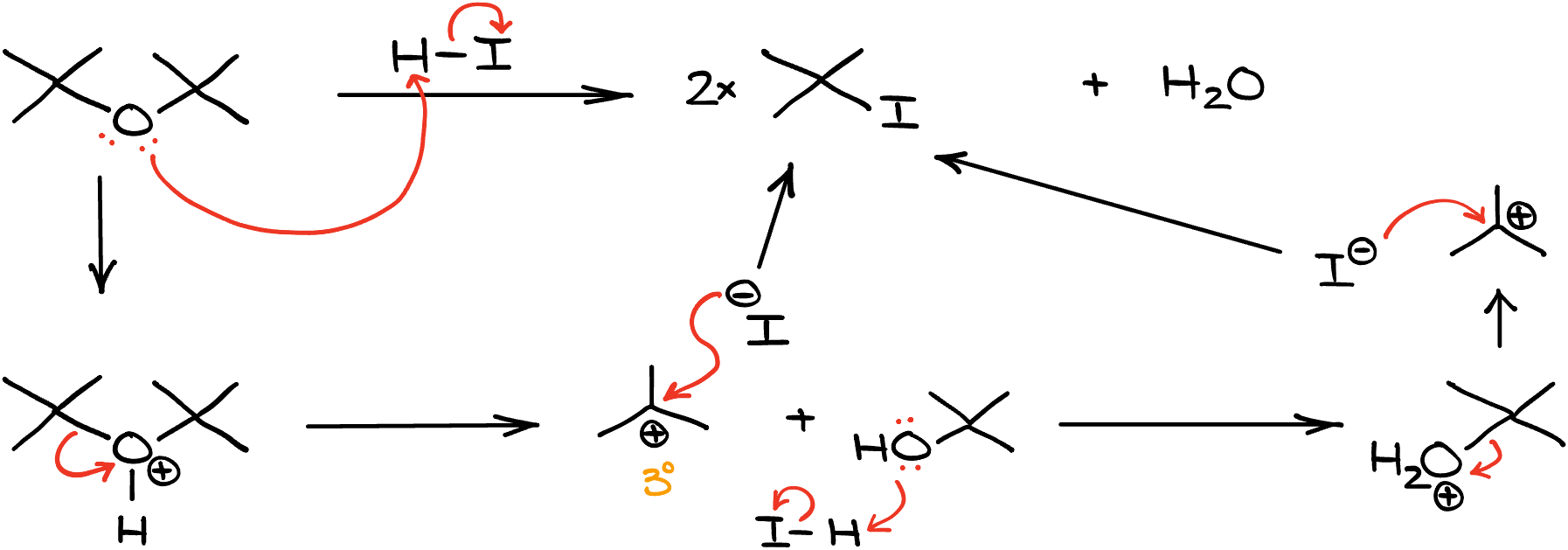
From the mechanism perspective, this reaction will start in the same way as the last one. We’ll first protonate the oxygen making a leaving group. It is irrelevant which side you’re going to treat as a leaving group, so go with whichever you like the most in this case.
Once we have our leaving group, the dissociation here won’t be an issue since tertiary carbocations are relatively stable and can form comparatively easily. And lastly, we’ll have the nucleophilic attack on the carbocation, giving us the first equivalent of the alkyl halide.
Then, we’re going to go into the second round of this reactions by protonating our alcohol, have water dissociate making a tertiary carbocation, and doing the nucleophilic attack making the second equivalent of the alkyl halide product.

And, of course, if you have a mixed ether, like for instance, this sec-butyl isopropyl ether, you’re going to make two different alkyl halides.
Trick to Predict the Products
Going through the mechanism for every reaction on the test is tedious. You still should know the mechanism for the test, that is always “a must.” But your instructor will also expect you to be able to predict the products fast without you having to draw the mechanism for each single reaction on the test.
So, luckily for us, predicting the product in the ether cleavage is quite easy.
- Step one: redraw your ether leaving some space around the oxygen.
- Step two: erase the oxygen.
- Step three: add halides to those bonds that used to be connected to the oxygen. Done!
Tricky Exam Questions
Now, being an organic chemist means that I, at least according to some of my students, hate fun. And while I strongly disagree with this statement, I do have to admit that we, organic chemists, like to throw a curved ball at our students from time to time on the test. So, there are a few common mean questions you might see on the test.
First, I’ll start with a more innocent one—cleavage of a cyclic ether. Remember, if we’re cutting through a cyclic molecule and we’re only doing so at one location, we’re not going to be making two products. Rather, we’re going to end up with a long chain.
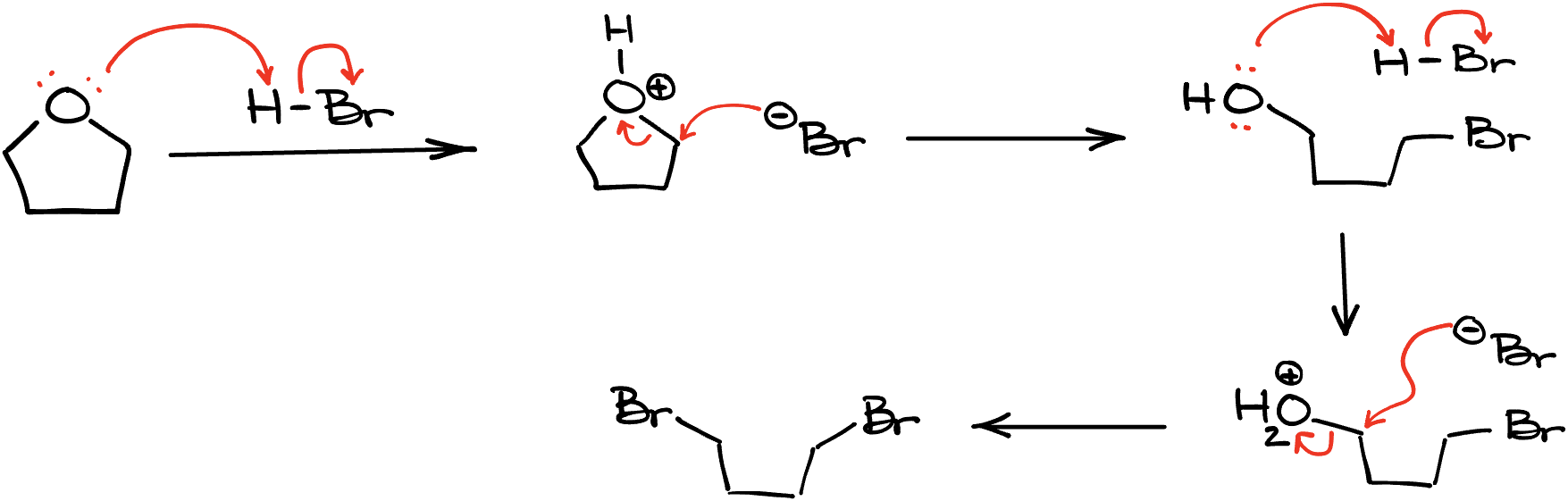
For instance, let’s look at this reaction of tetrahydrofuran with HBr. In this case, if we quickly go through the mechanism, we’ll get one long chain with two halogens sitting on it. If you need a little time to follow through the steps here, make sure you pause the video and study those steps carefully, so you can write a similar mechanism on the test if you need to. But the point here is to remember that you’re making a single product in this case, not two separate molecules.
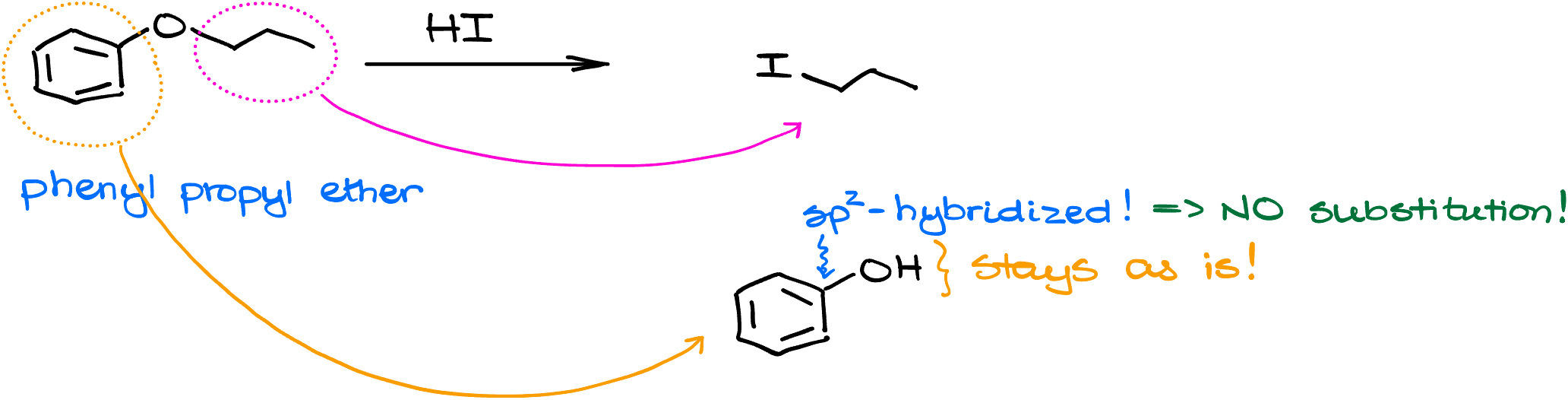
The other common tricky question that instructors love to throw at students on the test, is the cleavage of the phenyl ethers. For instance here, I have phenyl propyl ether. And let’s say, we’ll treat it with HI. In this case, the right side of the molecule will turn into a corresponding alkyl halide, 1-iodopropane. However, the left side of the molecule will stay with the -OH. Remember how you cannot easily do a substitution on the sp2-hybridized atoms? This is the case here as well. Phenol, my other product in this reaction, has its -OH group on the sp2-hybridized carbon. And therefore, it cannot be replaced with iodine via either an SN2 or an SN1 reaction. So, it stays as is.
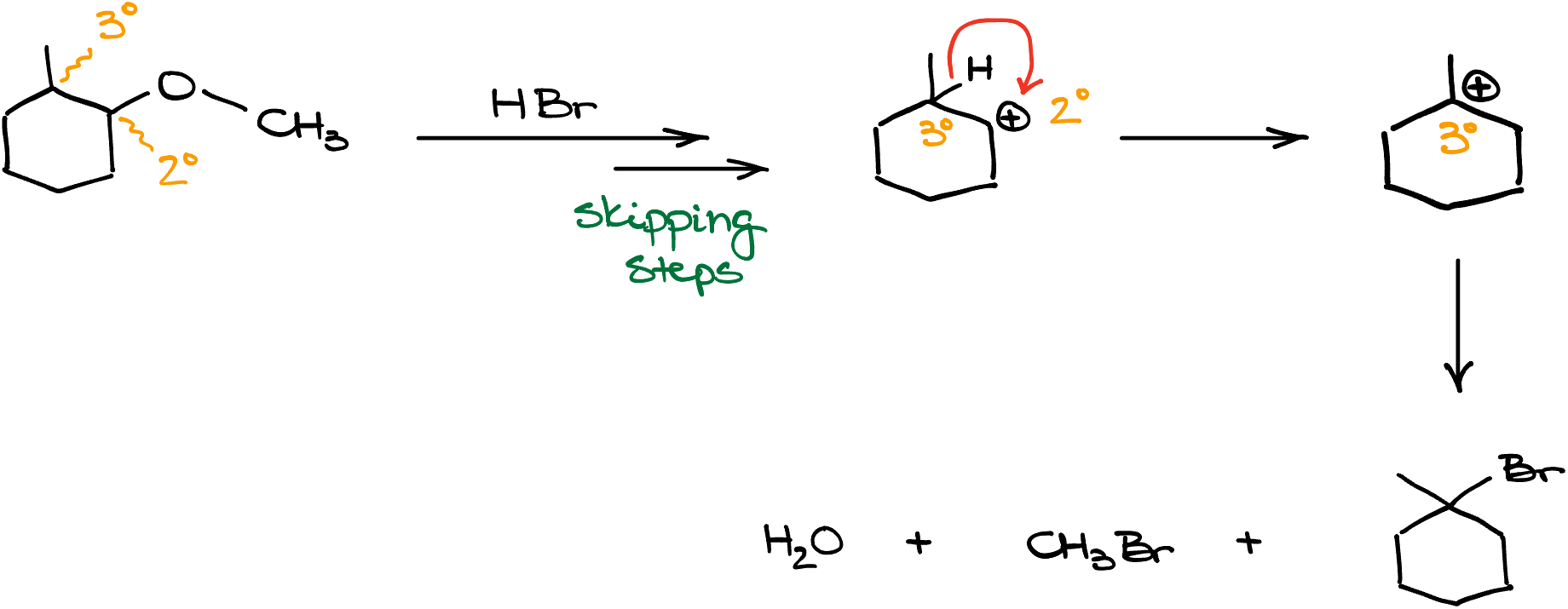
Finally, if we have secondary ethers, there can be a possibility of carbocation rearrangements. For instance, in this molecule have our oxygen attached to the secondary carbon which is right next to a tertiary position. So, if I were to cleave this ether with something like HBr, I’d end up with a secondary carbocation in the middle of my mechanism. And since this secondary carbocation is right next to a tertiary carbon, I’ll immediately see the carbocation rearrangement giving me a more stable carbocation. Remember, if a carbocation can rearrange to give you a more stable carbocation, it absolutely will! So, here, we’ll end up with the final product with the halogen on a completely different atom from where our oxygen used to be.
Concluding Thoughts
So, as you can see, we keep coming back to the same substitution and elimination reactions over and over again as we cruise through the chemistry of functional groups in organic chemistry. There’s a reason why we cover substitution and elimination reactions early in the course (or at least most instructors do), as it’s one of those topics that will keep on resurfacing throughout the course over and over again.
So, to recap what we have learned today:
- Ethers cleave with HX acids via either SN1 or SN2 mechanisms.
- Primary ethers cleave via the SN2 mechanism.
- Secondary and tertiary ethers cleave via the SN1 mechanism.
- If you have a secondary ether, beware of possible carbocation rearrangements.
- If you have the phenyl ether, leave it with the -OH group instead of the halide.
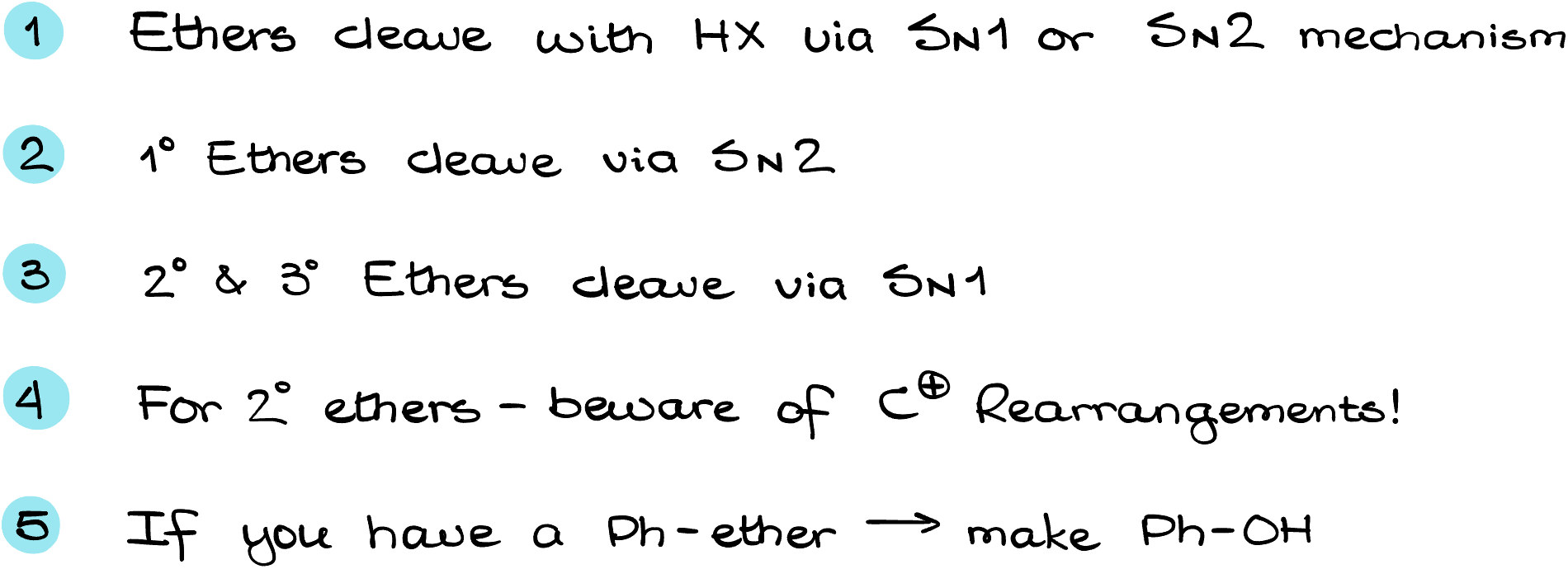
Cleavage of Cyclic Ethers
Many students struggle with the cyclic molecules, so let’s look through a few examples of how the ether cleavage looks like in the case of cyclic ethers.
