Hydrohalogenation of Alkynes
In this tutorial, we’ll explore the hydrohalogenation of alkynes, delving into the intricate mechanism of how alkynes react with hydrogen halides. We’ll highlight common examples that often appear in exams and, importantly, pinpoint frequent student mistakes to ensure you’re well-prepared to tackle this topic with confidence.
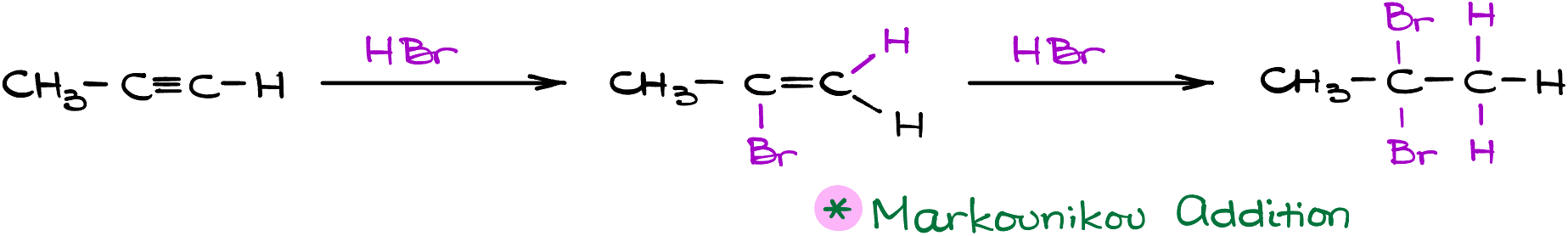
Mechanism of Hydrohalogenation of Alkynes: The Classic Textbook Approach
Diving into the hydrohalogenation of alkynes, textbooks typically illustrate this with common reactants like propyne and hydrogen bromide.
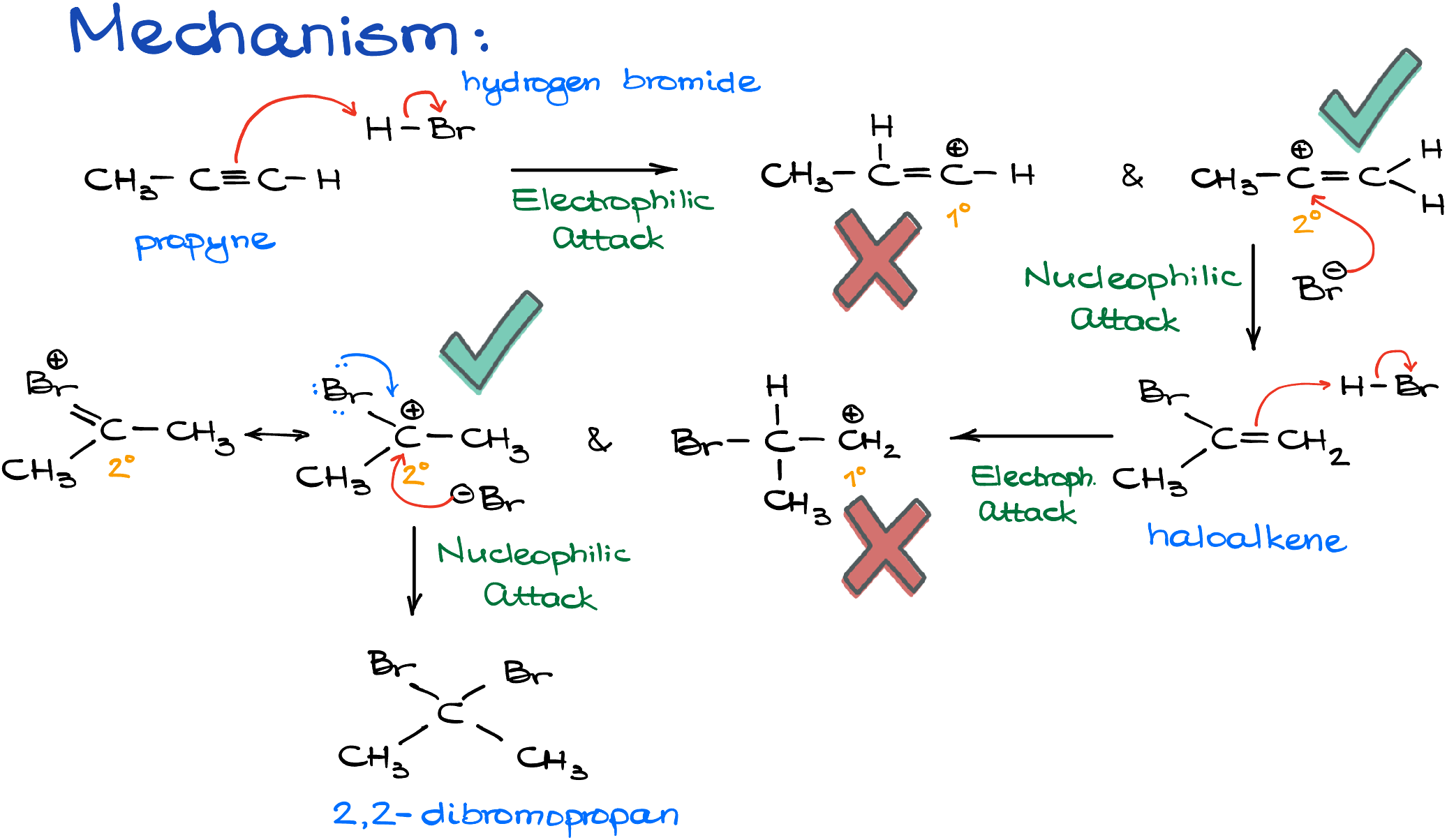
Here’s how the mechanism usually goes:
- Initial Electrophilic Attack: The alkyne, propyne in this case, undergoes an electrophilic attack by the hydrogen halide. This can lead to two different carbocations. While we could end up with a primary carbocation, it’s the secondary one that’s most likely formed due to its greater stability.
- Nucleophilic Attack: Following the formation of the secondary carbocation, a nucleophilic attack takes place, resulting in our first potential product.
- Continued Reaction: Should we decide to progress the reaction with an additional equivalent of the hydrogen halide, another electrophilic attack ensues. This once again gives rise to two carbocation possibilities: primary and secondary. With the secondary carbocation being favored, it’s further stabilized by resonance with the bromine atom, albeit weakly.
- Final Product Formation: The subsequent nucleophilic attack by the bromide ion finalizes the reaction, producing 2,2-dibromopropane. A key observation here is the Markovnikov’s rule in action, as the halide attaches to the more substituted carbon while the hydrogen chooses the less substituted carbon. A neat and predictable pattern, right?
Markovnikov’s Rule: A Quick Overview
Markovnikov’s rule, proposed by the Russian chemist Vladimir Markovnikov in 1870, predicts the outcome of the addition of protic acids (like HCl, HBr, and HI) to unsaturated hydrocarbons, such as alkenes or alkynes.
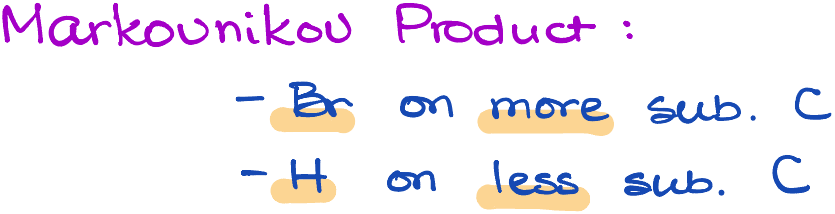
According to the rule:
When a protic acid is added to an alkene, the acid’s hydrogen (H) will attach to the carbon with the highest number of hydrogen atoms already attached to it.
Conversely, the non-hydrogen part of the acid (e.g., the Cl, Br, or I) will add to the carbon with the fewest hydrogens.
This rule helps us anticipate the major product in many addition reactions. One of the underlying reasons for this pattern is the stability of the carbocation intermediates formed during the reaction; more substituted carbocations are generally more stable than less substituted ones.
The Curious Case of 3-methylbut-1-yne and HBr
When reacting 3-methylbut-1-yne with HBr, the process unfolds as follows:
- Electrophilic Attack: HBr attacks the pi-bond.
- Formation of Carbocations: Two carbocations are possible – a primary one and a secondary one. Given the sp-hybridization, the secondary carbocation sports a linear geometry with 180° bond angles.
- Secondary Carbocation Preference: The primary one doesn’t really stand a chance, so the story continues with the secondary carbocation.
- Nucleophilic Attack: Bromide anion steps in, leading to our product, 2-bromo-3-methylbut-1-ene.
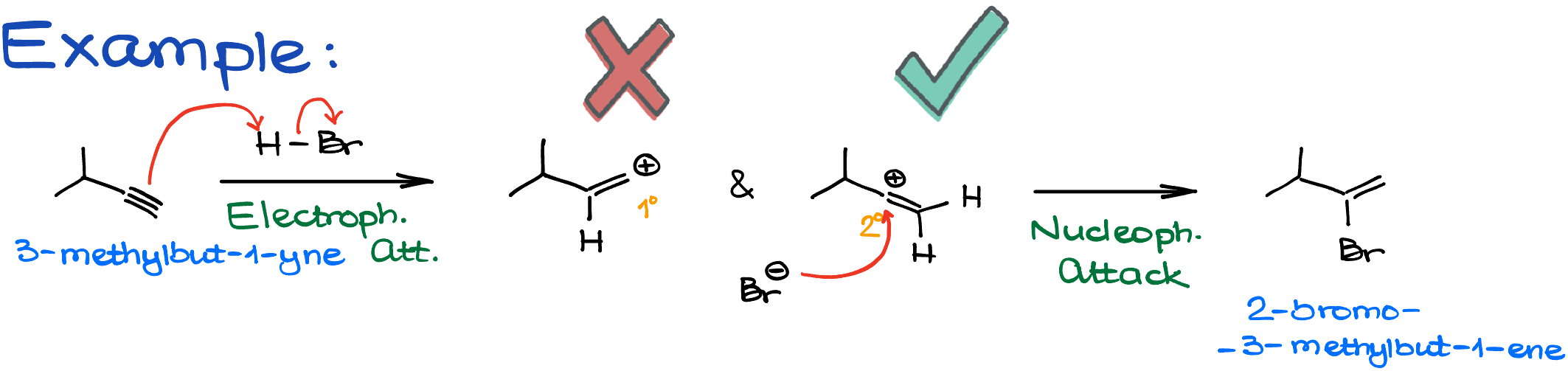
Now, here’s where things get spicy! You might be scratching your head, wondering, “Why no carbocation rearrangement?”
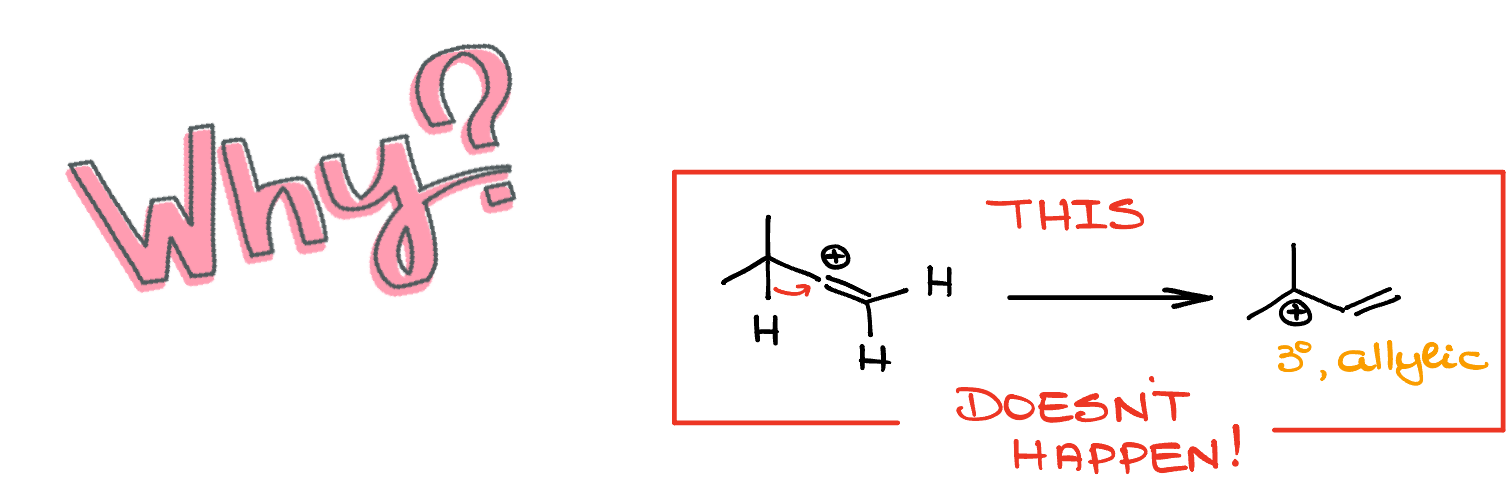
Especially since a tertiary allylic carbocation is a far more stable option. Well, despite what the textbooks might hint at, not everything follows the beaten path. And believe it or not, sometimes, the mechanisms we’re taught aren’t the whole story. Remember, every quirk in chemistry has its reason, even if it momentarily feels like…well, let’s just say it’s not always as it seems!
Termolecular Mechanism: When Experimental Data Speaks Louder
Often in sophomore organic chemistry, students learn a mechanism that doesn’t quite align with experimental data. Let’s set the record straight!
Concerted Termolecular Mechanism: The crux of the matter is that recent kinetic studies reveal a termolecular mechanism at play. Imagine an alkyne molecule having a chat with two separate HBr molecules. The result? Our product forms directly, side-stepping any carbocation shenanigans. With no carbocation middleman, there’s zero chance of carbocation rearrangements. If you’ve ever stumbled across a study that proves otherwise or gives a reason (beyond the magic wand effect) for a non-rearranging carbocation, drop a comment below. I’d love a good scholarly surprise!
The Second Step – Just Regular Hydrohalogenation: Moving on, the next phase is textbook hydrohalogenation. Why no carbocation gymnastics here? It’s all thanks to our bromine buddy! The bromine’s electron pairs lend some resonance support, ensuring our intermediate stays stable and predictable. A straight path, with no twists or turns!
Another Strong Case for Termolecular Mechanism
Support continues to mount for the termolecular mechanism, and a reaction between but-2-yne and HBr.
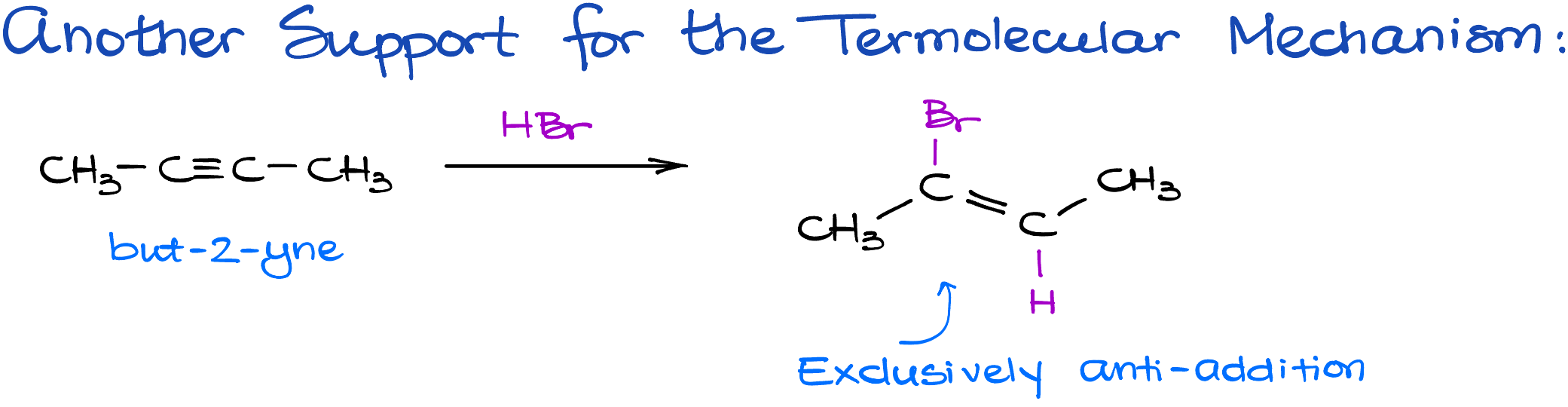
Here’s the catch: The midway product of this reaction is overwhelmingly the outcome of an anti-addition. If a carbocation strutted its stuff here, we’d spot both products since carbocations don’t dance to a single stereochemical beat. But guess what? We only see one product. That’s a tell-tale sign!
Know Your Classroom Context
Two mechanisms stand at a crossroads: the familiar one we share with students (lacking substantial experimental backing) and the more realistic termolecular version. Here’s a golden nugget of advice: Understand your professor’s expectations. A handful of textbooks touch on the termolecular nature. If yours isn’t among them, you’re likely learning the carbocation route. So, before you dive deep, have a chat with your instructor. After all, they hold the grading pen, not me!
