Nomenclature of Carbohydrates (the Fundamentals)
While carbohydrates are not really any different from other organic molecules, they do have their own nomenclature of carbohydrates. Majorly, this is the result of the historic names given to many sugars. And as history would have it, those names kinda stuck and became the “norm” that you now need to know for your exams.
As all carbohydrates have the same general molecular formula, Cn(H2O)m, we are going to focus on the following structural aspects of the molecules:
- The molecule size: how many carbons you have in the molecule
- The type of a functional groups you have in the molecule: an aldehyde or a ketone in addition to alcohols
- The open-chain vs cyclic structure
Naming the Carbohydrate Length
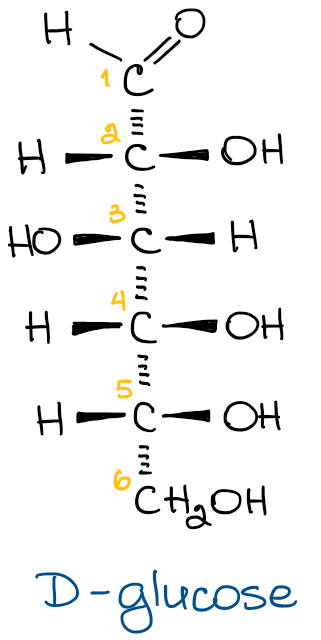
The simplest carbohydrate has 3 carbons. We use the greek numerals to call the number, aka tri-, tetra-, penta-, hexa-, and add the ending -ose to denote that it’s a carbohydrate. For instance, a triose is a carbohydrate with 3 carbons, while hexose is a carbohydrate with 6 carbons in the molecule. For instance, the glucose is an example of a hexose because it has six carbons in the molecule.
This type of a name, however, doesn’t tell us the exact nature of the molecule. For instance, there are 24 different hexoses (12 of which exist in nature). This list includes glucose, galactose, fructose, mannose, etc. So, when I say that we’re dealing with a hexose, that doesn’t mean much except for the fact that the molecule contains 6 carbons.
Naming the Major Functional Group in a Carbohydrate
Sugars, or carbohydrates, have two major functional groups: an aldehyde or a ketone (both are collectively called carbonyls), and an alcohol functional group. Carbohydrates generally have multiple alcohol functional groups, so we never focus on those. However, sugars will only have one aldehyde OR one ketone functional group. We specify this in the name by adding aldo– or keto– prefix to the carbohydrate name. As you can guess, aldo- goes together with an aldehyde, and keto- with the ketone-containing carbohydrates.
Here are some examples of aldoses:
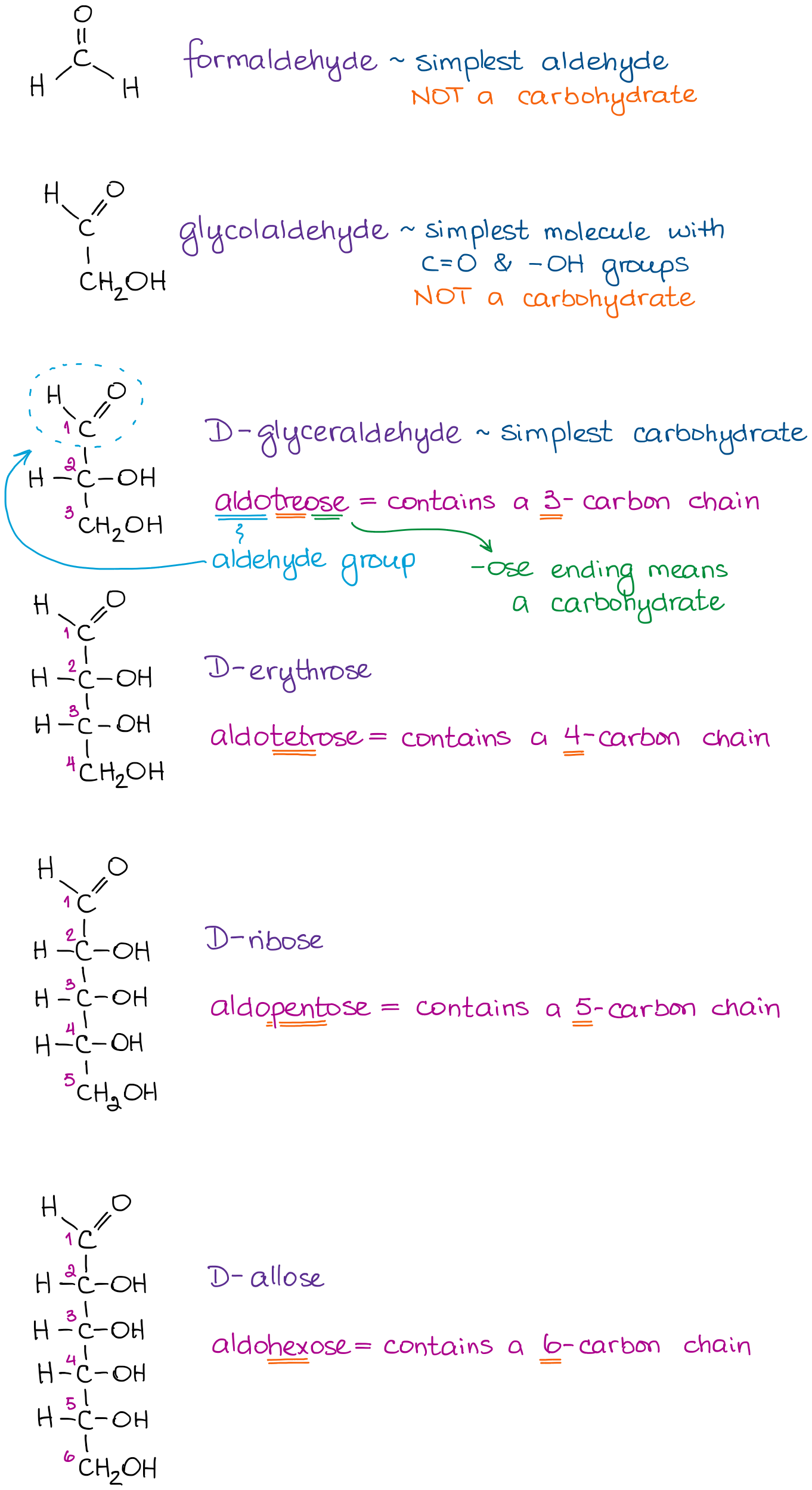
As you can see from these examples, we start the name by saying that the molecule is an aldehyde using the aldo- prefix. We then say how many carbons are there in the molecule. And we finish by adding the -ose ending to specify that it’s a carbohydrate we’re dealing with.
We can do the same for ketoses. As ketoses contain a ketone functional group, we obviously cannot have it at the beginning of the chain. Most common ketoses have a ketone functional group on the second carbon in the chain.
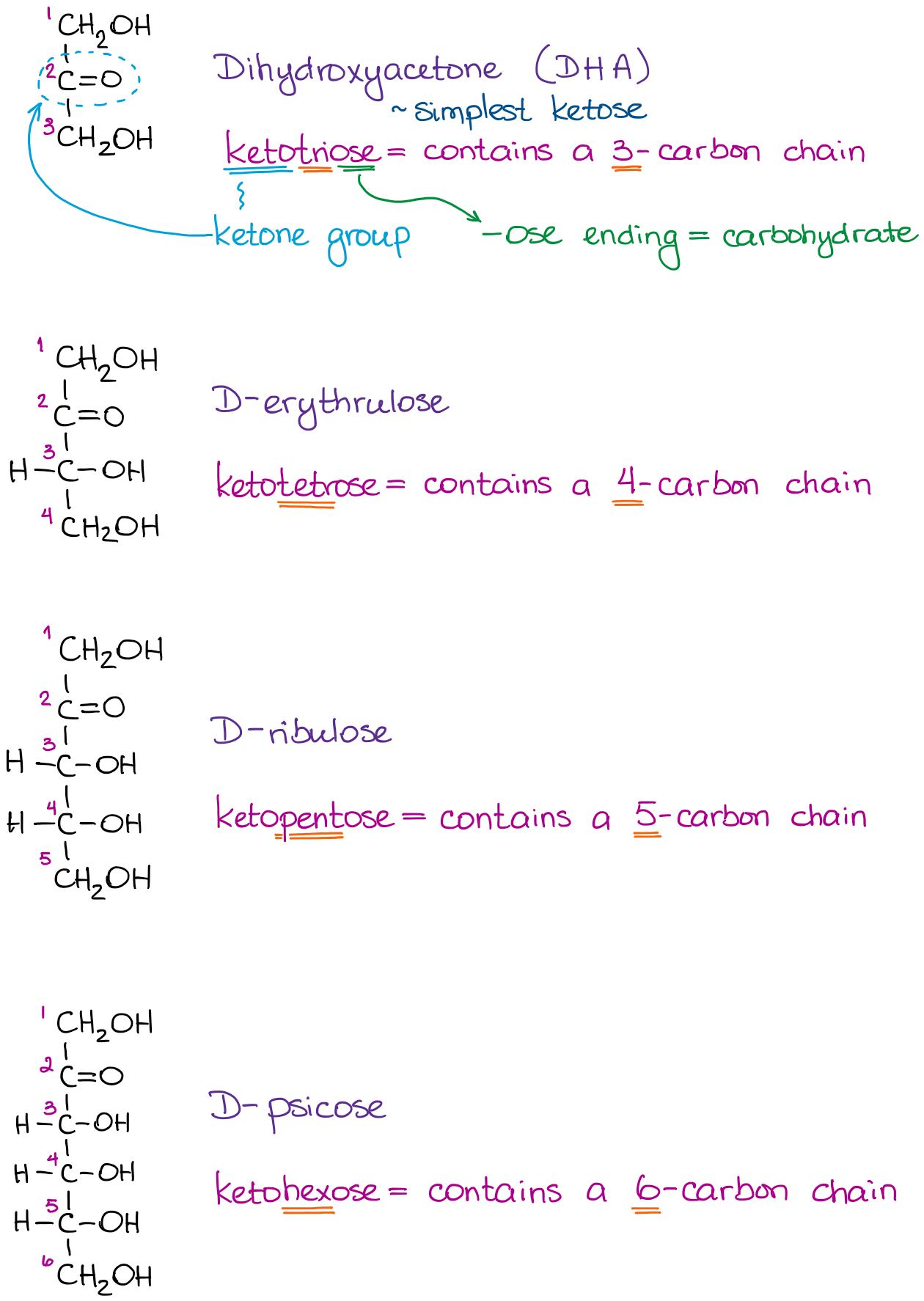
The nomenclature of ketoses follows the same principles as for aldoses: you start by saying keto- to point out the functional group. Then, say how many carbons you have in the molecule and add -ose to signify the carbohydrate.
Stereochemistry of Carbohydrates
Carbohydrates have multiple stereocenters. The simplest one, glyceraldehyde, only has one. However, as you go down the line to more complex carbohydrates, you get more and more stereocenters. So, for a molecule like glucose, you have 4 chiral carbons. This means that glucose has a grand total of 16 stereoisomers! Number of stereoisomers = 2n , where “n” is the number of chiral centers. Each of those molecules has its own unique name! Most instructors, however, won’t require you to actually memorize all of the structures. It might be a good idea, though, to commit a few common ones to memory. Such common carbohydrates are glucose, mannose, galactose, and fructose. You’ll see them all the time in the biological pathways (especially, glucose), so it’ll make your life easier if you can recognize those.
D and L Configurations
One piece of stereochemistry that we do specify is the configuration of the last chiral carbon in the chain. We use the letters D and L for this purpose. Why not stick to the good ‘ol R and S? Because of the traditional naming convention that existed before the Cahn-Ingold-Prelog system. As I’ve mentioned earlier, carbohydrates, like many biomolecules, have been known for quite some time and have acquired their own naming system that got stuck with us. D and L configuration refers to the last chiral carbon in the molecule:
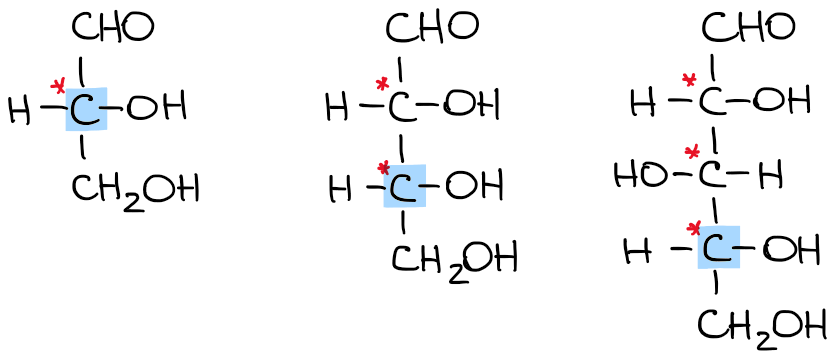
A molecule with the -OH group to the right is assigned the D-configuration. Likewise, the molecule with the -OH group on the left has the L configuration.
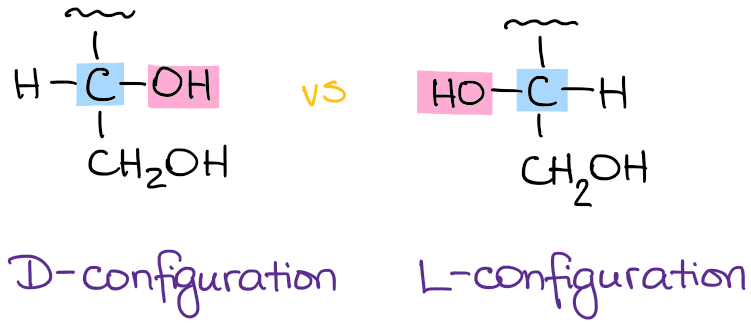
The meaning behind the letters is: “D” stand for the “dextrorotating” while “L” means “levorotating” molecule. D & L configurations DO NOT ACTUALLY correspond to the rotation of the plane polarized light! This relationship ONLY holds for glyceraldehyde. A D-sugar can be both (+) and (-), likewise, L-sugar can be either as well. Just like R and S, D and L are merely the way we call the molecules and they have no relation to actual physical properties.
All biologically-relevant carbohydrates have D-configuration.
How D and L Relate to R and S?
The strict IUPAC rules always ask for the R and S configurations for the stereocenters. If you ever need to assign the R and S to a carbohydrate, first, you gotta remember that in the Fischer projection all vertical lines are the “dashes” while all horizontal lines are the “dashes.”
Let’s assign R and S to glyceraldehyde:
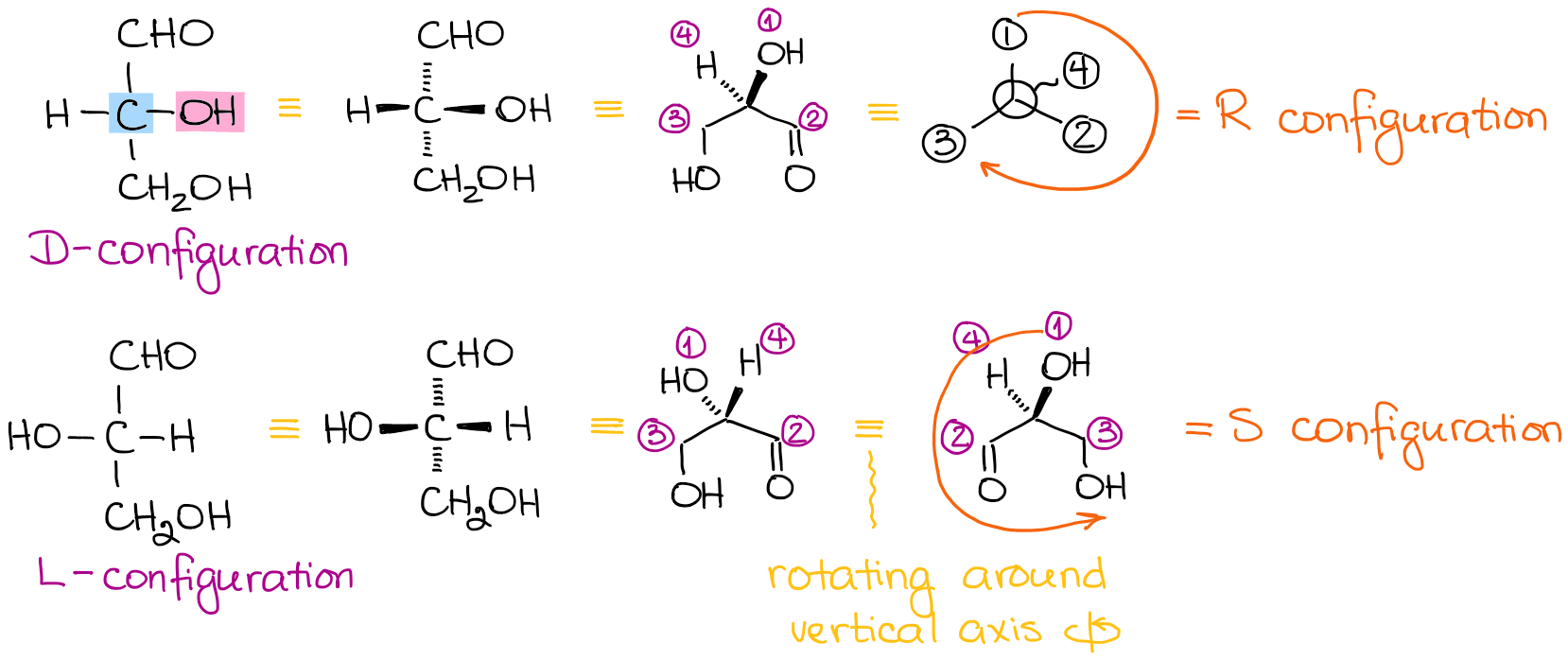
As a rule of thumb, you can remember that D sugars are R and L sugars are S. However, remember, that this shortcut only works for the open chain common sugars. So, make sure you actually know how to assign R and S for molecules.
IMPORTANT: D and L carbohydrates are enantiomers.
It’s a very common misconception that the difference between the D and L sugars is only in the last carbon’s stereochemistry. THIS IS INCORRECT! The D and L sugars with the same name are actually enantiomers of each other.
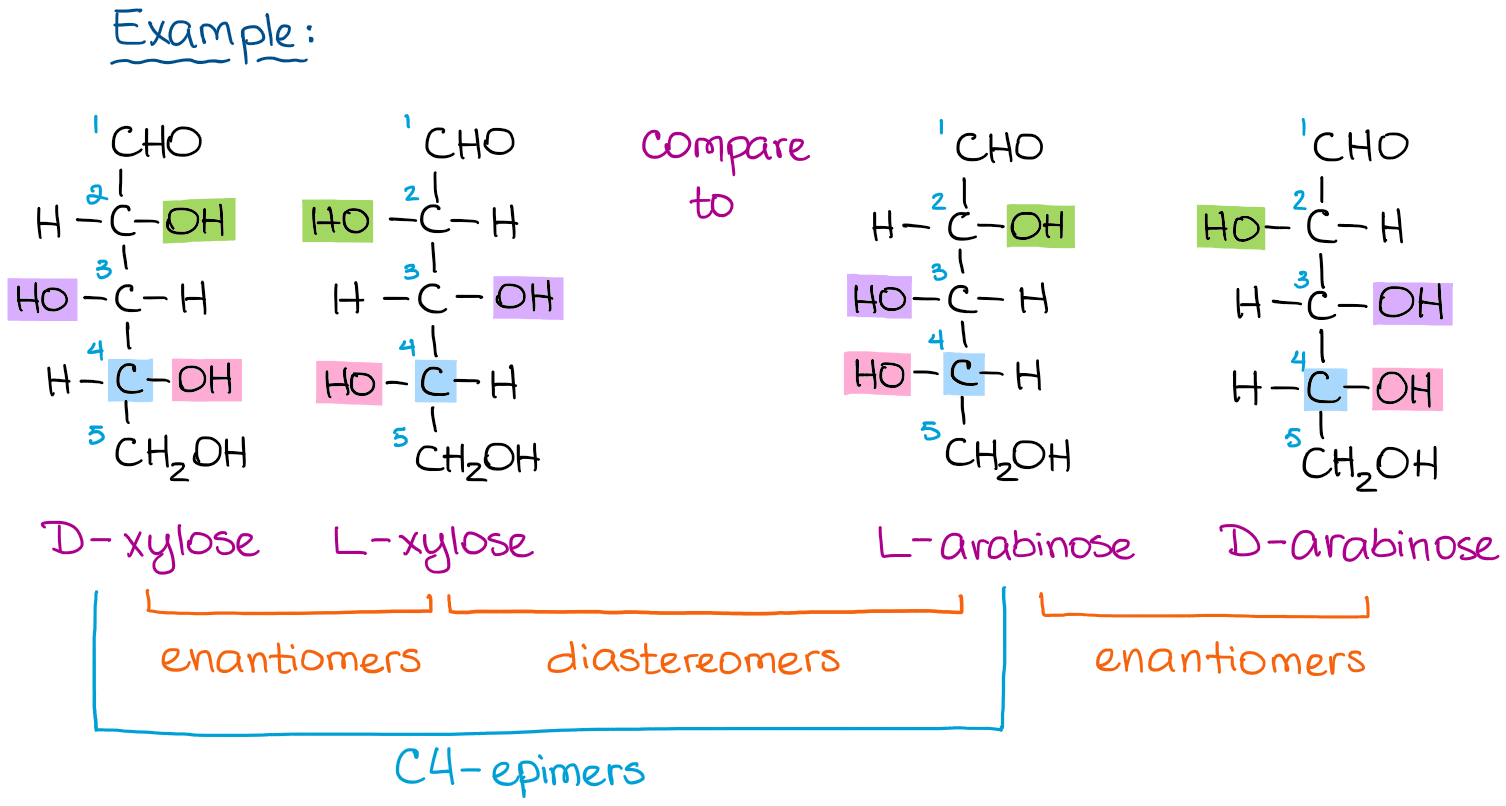
The example above illustrates this point. If we look at the D- and L-xylose, they are non-superimposable mirror images, which is enantiomers. However, if you only change the last stereocenter in D-xylose, you get L-arabinose and NOT L-xylose. Stereoisomers that only differ in one stereocenter configuration are called epimers. Thus, D-xylose and L-arabinose are C4-epimers since they are different in the 4th carbon.
Cyclic Forms of Carbohydrates
Nomenclature of carbohydrates also includes the naming the cyclic forms. Since carbohydrates contain a carbonyl and an alcohol functional groups, they can form intramolecular (cyclic) hemiacetals. A carbohydrate must be at least a tetrose to do that, so intramolecular cyclic forms don’t exist for smaller carbohydrates.
Here’s an example of a common sugar D-galactose forming two different cyclic forms:
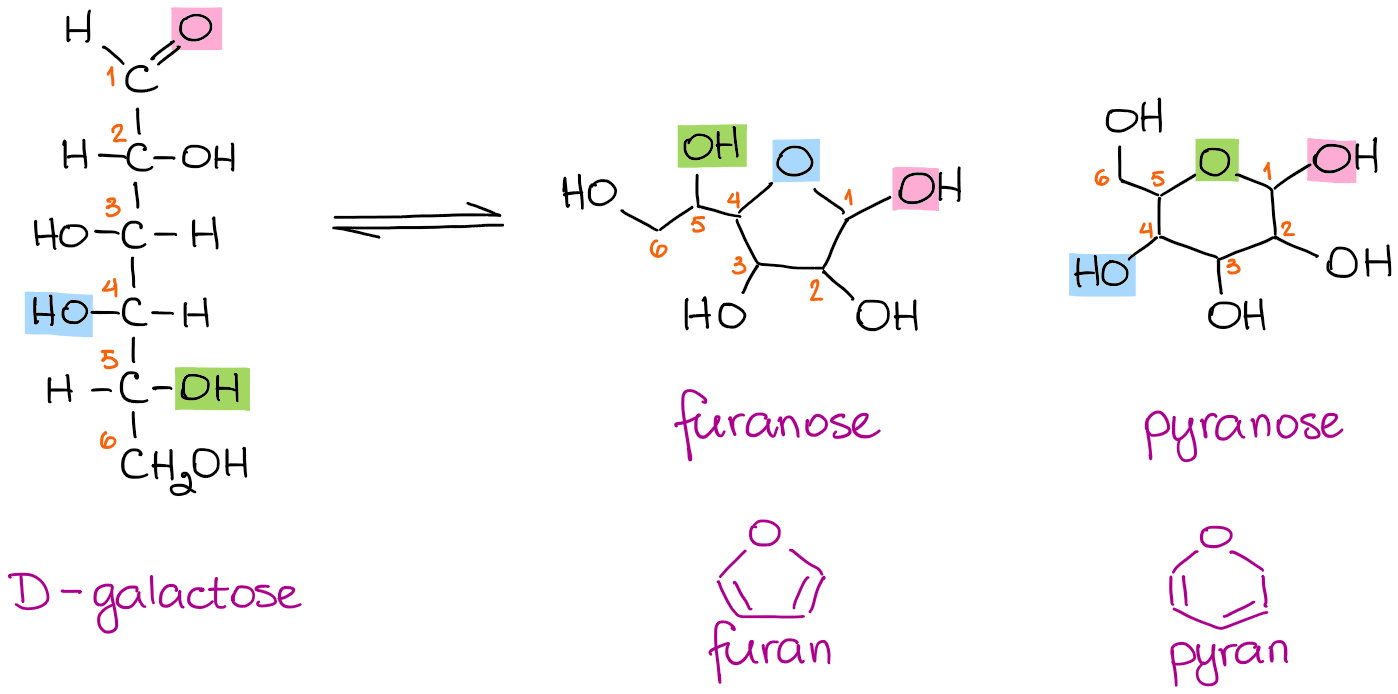
When the -OH on the 4th carbon is participating in the cyclization, you get a 5-membered ring. When the 5th carbon provides the -OH, you get a 6-membered ring. Due to the analogy with the common oxygen-containing heterocyclic compounds furan and pyran, the 5-membered rings are called furanoses, and 6-membered rings are called pyranoses.
There’s a whole process to this equilibrium and the process of converting between the open-chain and the cyclic forms. This article, however, is about the nomenclature, so I’ll talk about converting between the Fischer, Haworth, and chair conformations some other time.
Haworth Projections
We often use the special type of drawing to depict the cyclic forms of carbohydrates. We call them Haworth Projections or forms. Basically, a Haworth projection is a cyclic structure with, traditionally, carbon #1 to the right and the bottom portion of the structure oriented towards the observer.
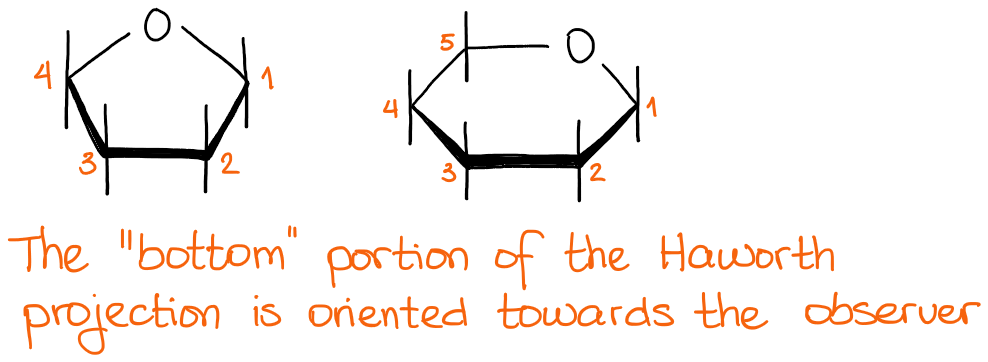
C1 in a cyclic carbohydrate is called anomeric carbon. This carbon used to be a carbon of the C=O in the open-chain structure before the cyclization. Anomeric carbon is special because it doesn’t have a set stereochemistry and can be in an α-form or a β-form. The α- and the β-forms are defined as trans or cis isomers of the cyclic carbohydrates where we look at the anomeric -OH and the carbon #5 or #6 for furanoses or pyranoses correspondingly.
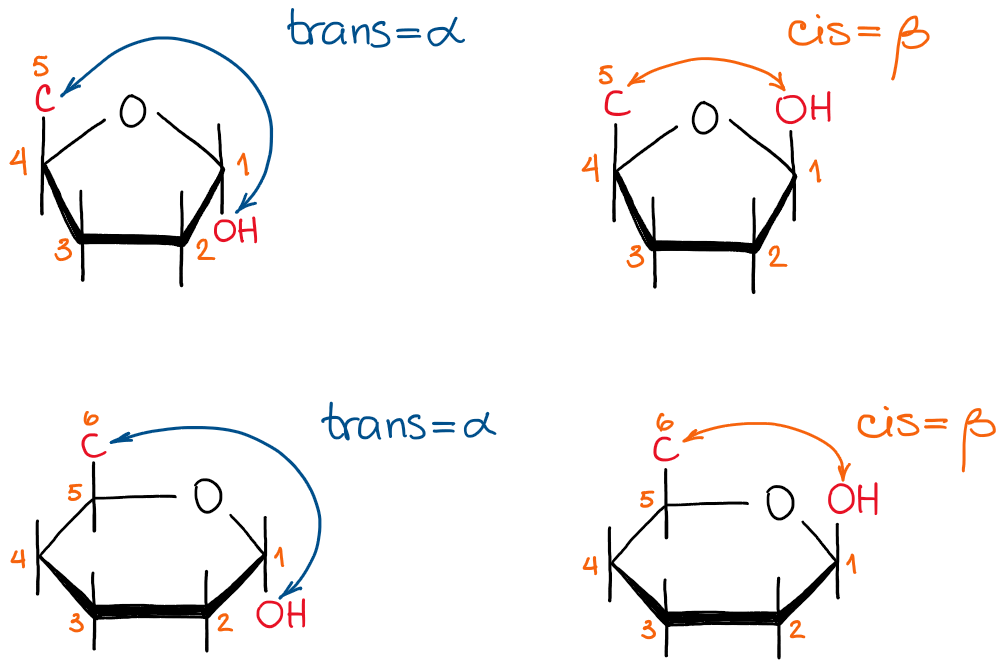
The α- and the β-forms of furanoses and pyranoses
The stereochemistry of the other carbons in the molecule is irrelevant.
This pretty much summarizes the nomenclature of carbohydrates and the major terms you need to know to ace your tests. Let me know if the comments below if you’d like to see more examples or similar posts!

Good lecture
yeah i really enjoyed the lecture and help to know more the biomolecule structures and naming them
I really enjoyed the lecture and I wish to get more for proteins, lipids, vitamins and enzymes.
I’m planning to add some more biochemistry related materials later this semester, but it’s not going to be my focus for a while.
This one is best website I give this five stars♥️