Hydrolysis of Nitriles
Nitriles are quite easy to make via a simple SN2 reaction making them a useful intermediate in the carboxylic acid synthesis. And in this tutorial, I wanna talk about how exactly to convert nitriles into carboxylic acids. And BTW, this is one of the instructors’ favorite exam questions and I’ve seen it on exams millions of times! The mechanism is somewhat long which means it’s easy to miss a step or do something wrong. So, let’s look at this reaction in detail so you’re all prepared for the next test.
Nitrile Hydrolysis Overview
Nitriles can be hydrolyzed on both acidic and basic conditions. Acidic conditions typically yield carboxylic acids, while the basic conditions can give you either carboxylic acids or amides.
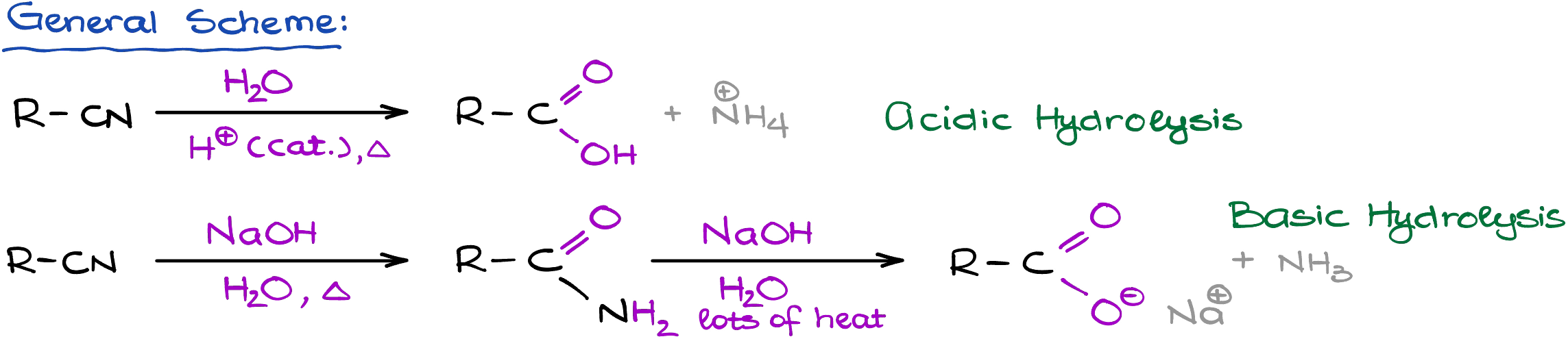
And the reason why I say “typically” is because it all depends on the conditions of the reaction and how vigorous those conditions are. If you perform your reaction in milder basic conditions, you will typically end up with an amide. However, harsher conditions (higher temperature, vigorous reflux over extended periods of time) typically give carboxylic acids. Also, you’re likely to get the carboxylic acid in acidic conditions regardless of how vigorous your conditions are.
Mechanism of the Acidic Hydrolysis of Nitriles
In acidic conditions, reaction starts with the protonation of the nitrile making it more electrophilic. Since nitriles are at the very bottom of the reactivity scale of carboxylic acid derivatives, you really need to make them more reactive to interact with a poor nucleophile like water.
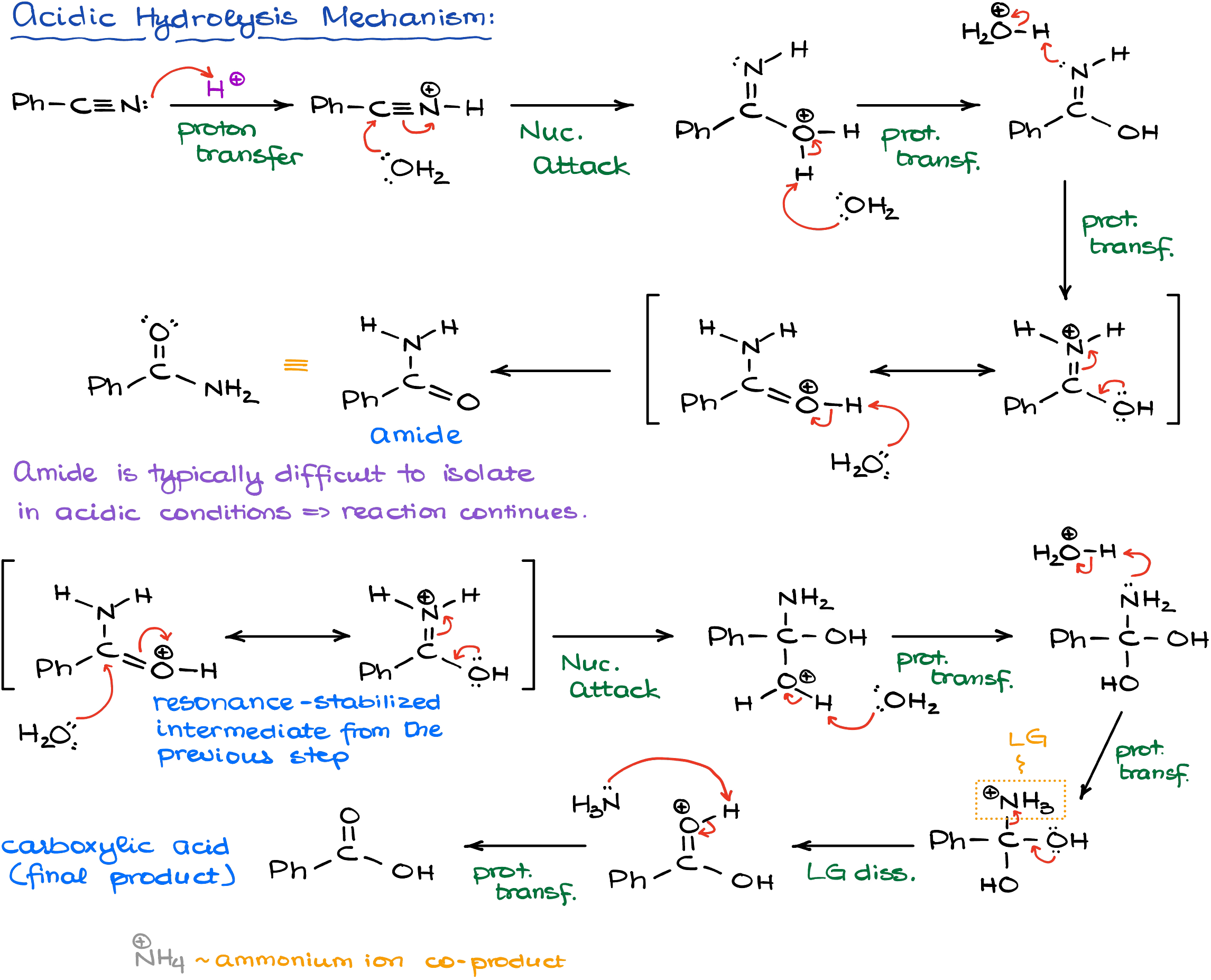
Once we are done with the initial nucleophilic attack, we’ll go through the series of proton transfers to make an amide. And as I’ve mentioned earlier, this is typically not the end of this reaction in acidic conditions. We’ll protonate our amide again and do another acyl substitution replacing the nitrogen altogether.
Since we work in acidic conditions, the ammonia (our co-product of the last step) will get immediately protonated rendering it completely non-nucleophilic. This last step is the driving force for the entire reaction as it’s just impossible to go back from this point.
Mechanism of the Basic Hydrolysis of Nitriles
In the basic conditions, however, the reaction starts with the immediate nucleophilic attack by the hydroxide.
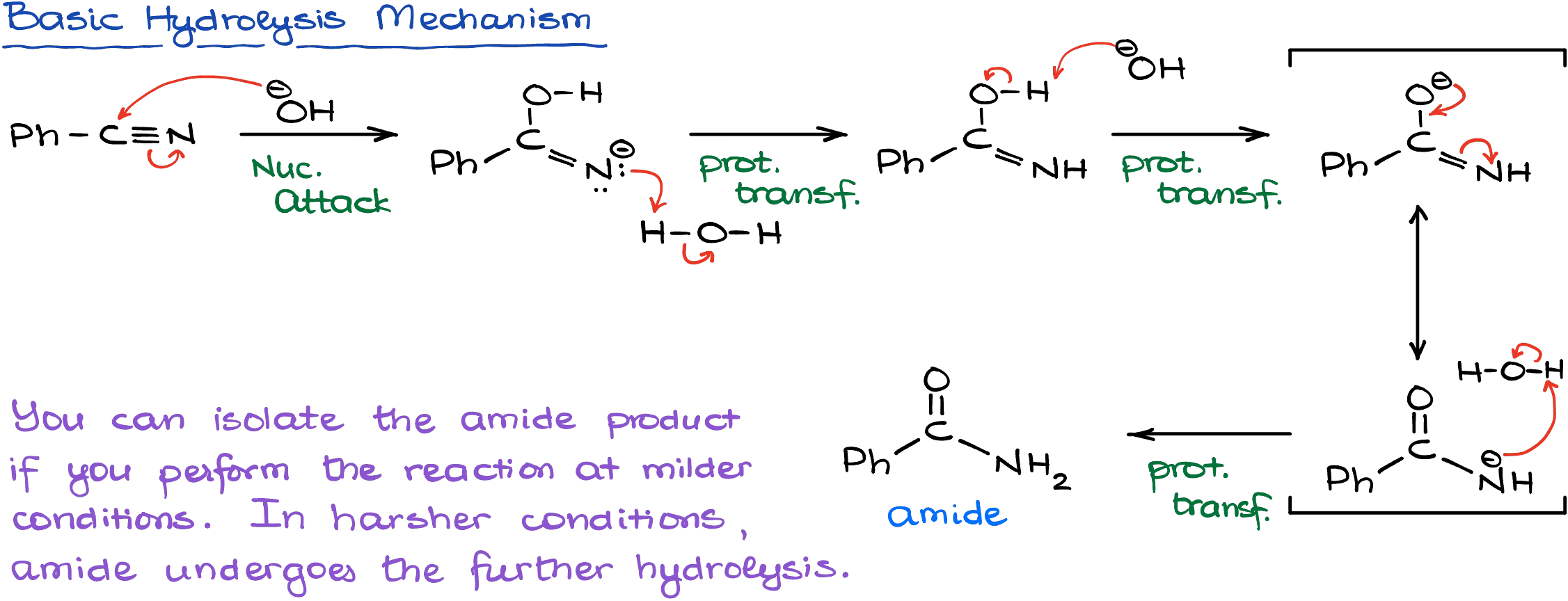
Since many steps in this mechanism yield negatively charged nitrogen intermediates, most of the steps here are quite unfavorable requiring significantly harsher conditions than the acidic hydrolysis.
Interestingly, once we get the amide, we can potentially stop the reaction at that point and isolate our amide as the final product. However, if we increase the temperature and subject our reaction mixture to a continuous vigorous reflux, we’ll push the reaction to the next phase, which is the acyl substitution replacing the nitrogen-containing part with the hydroxyl.
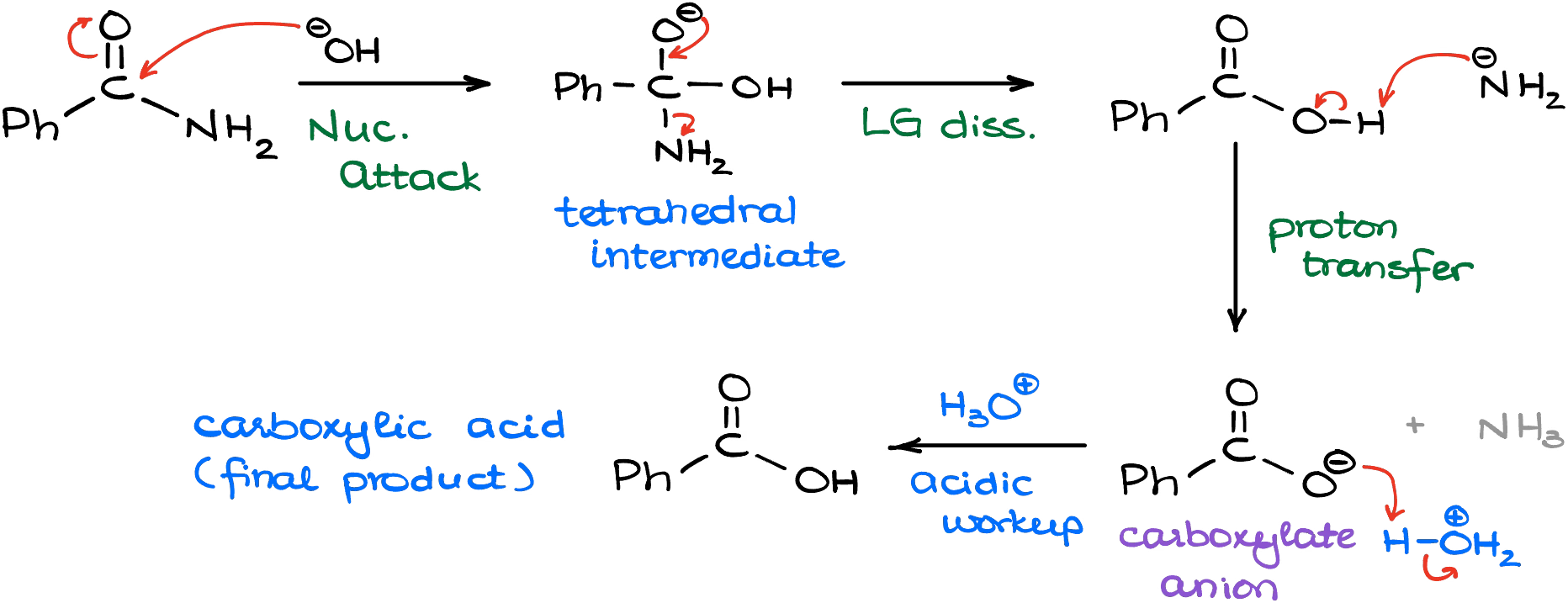
In this case, we’ll make a carboxylic acid and an amide (NH2–) co-product. Yes, we use “amide” as the name for the carboxylic acid derivative and for the conjugate acid of ammonia. It indeed can be confusing, but bear with me here. And since the amide (NH2–) is incredibly basic, it will immediately snatch the proton of the carboxylic acid making a corresponding carboxylate. Thus, if you want to make your carboxylic acid, you’ll need an extra step to protonate your carboxylate.
Concluding Thoughts
So, while these mechanisms can be a little intimidating due to how long they are, they are quite manageable with a little practice. I do encourage you to practice these two mechanisms a few times as they are quite popular among the instructors, and I often see them on the tests and finals.
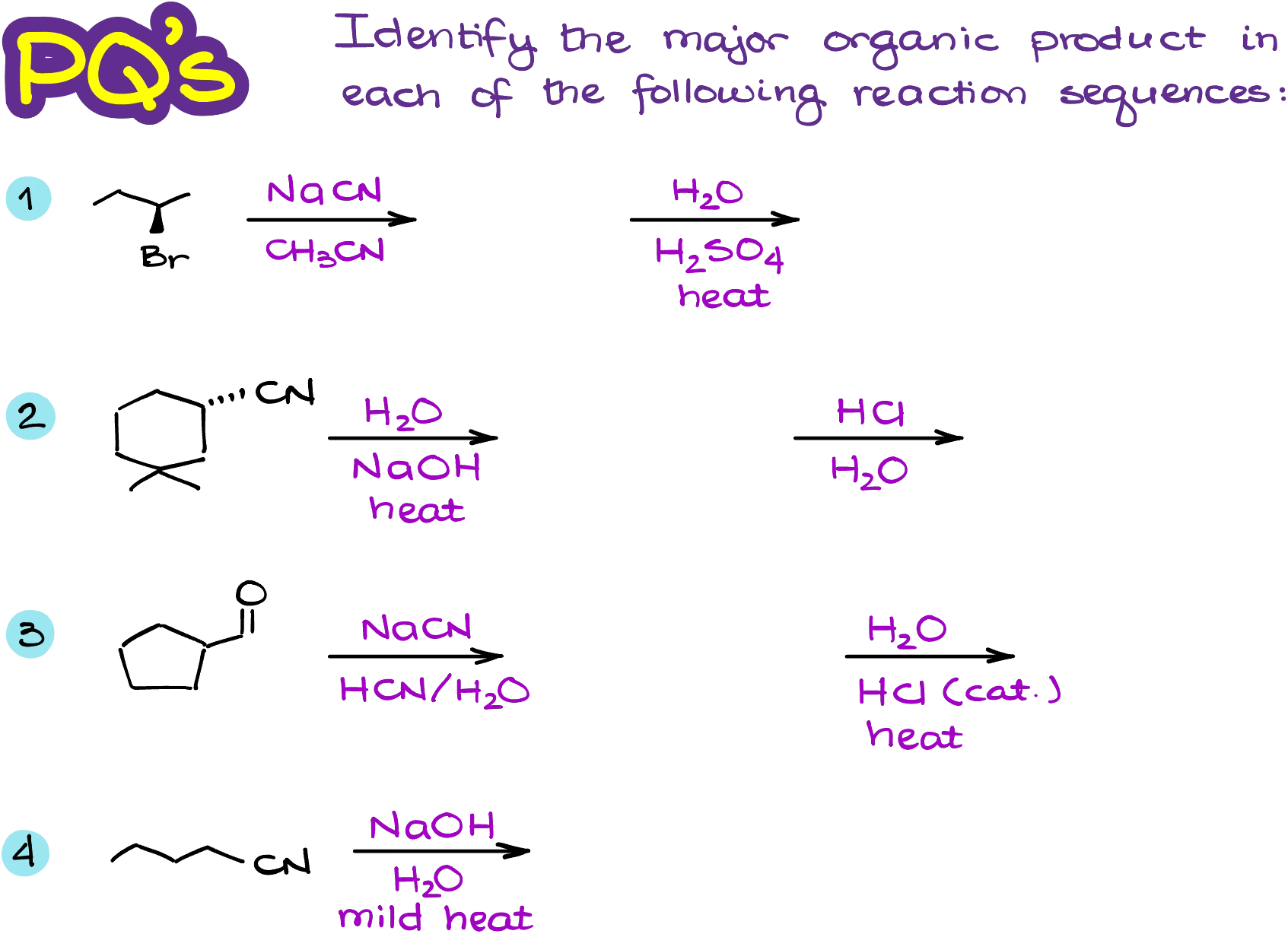
Would you like to see the answers and check your work?
Sign up or login if you’re already a member and unlock all members-only content!
