Fischer Projections
Let’s dive into the world of Fischer Projections!
What are Fischer Projections?
Fischer Projections are a method of representing 3D molecules in a 2D format.
Understanding the Basics:
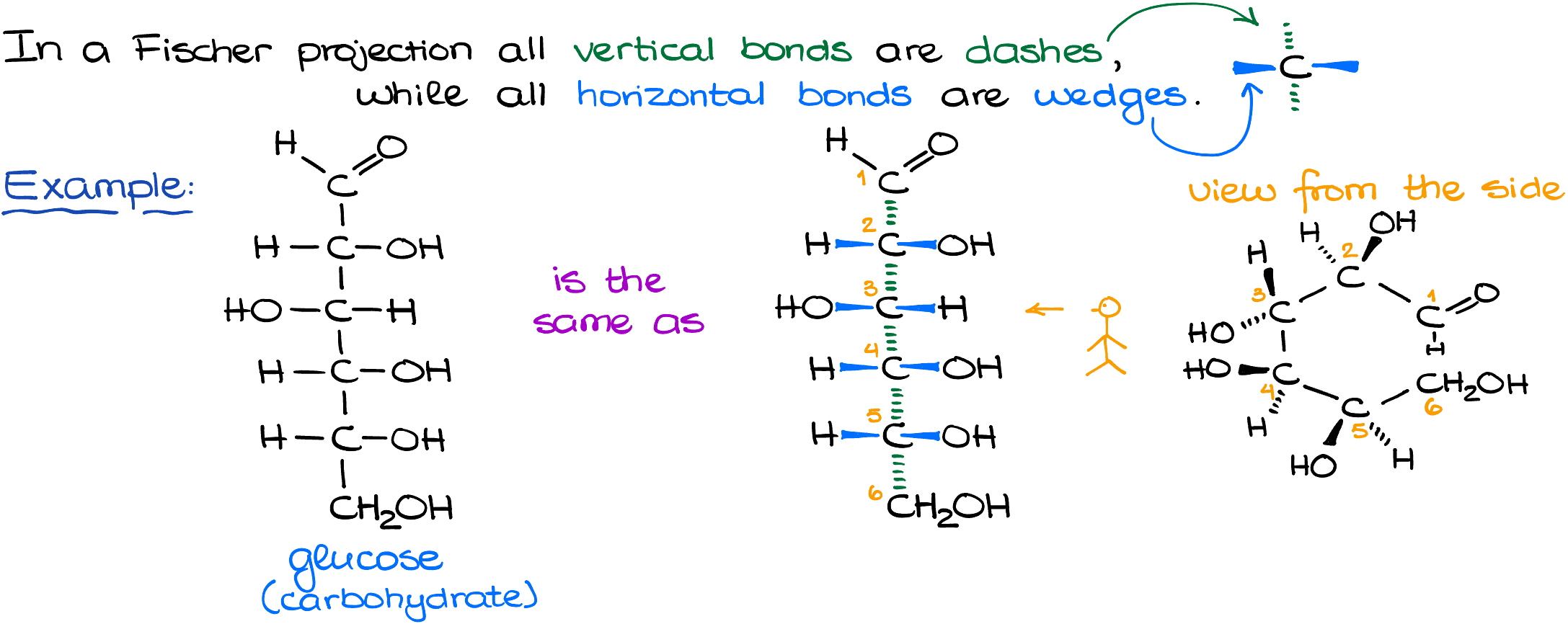
- Vertical Bonds: Represent bonds going away from you.
- Horizontal Bonds: Represent bonds coming towards you.
Imagine standing in front of a molecule with arms stretched out. The vertical lines are like the molecule’s arms reaching behind it, while the horizontal lines are reaching out to greet you!
Illustrative Examples:
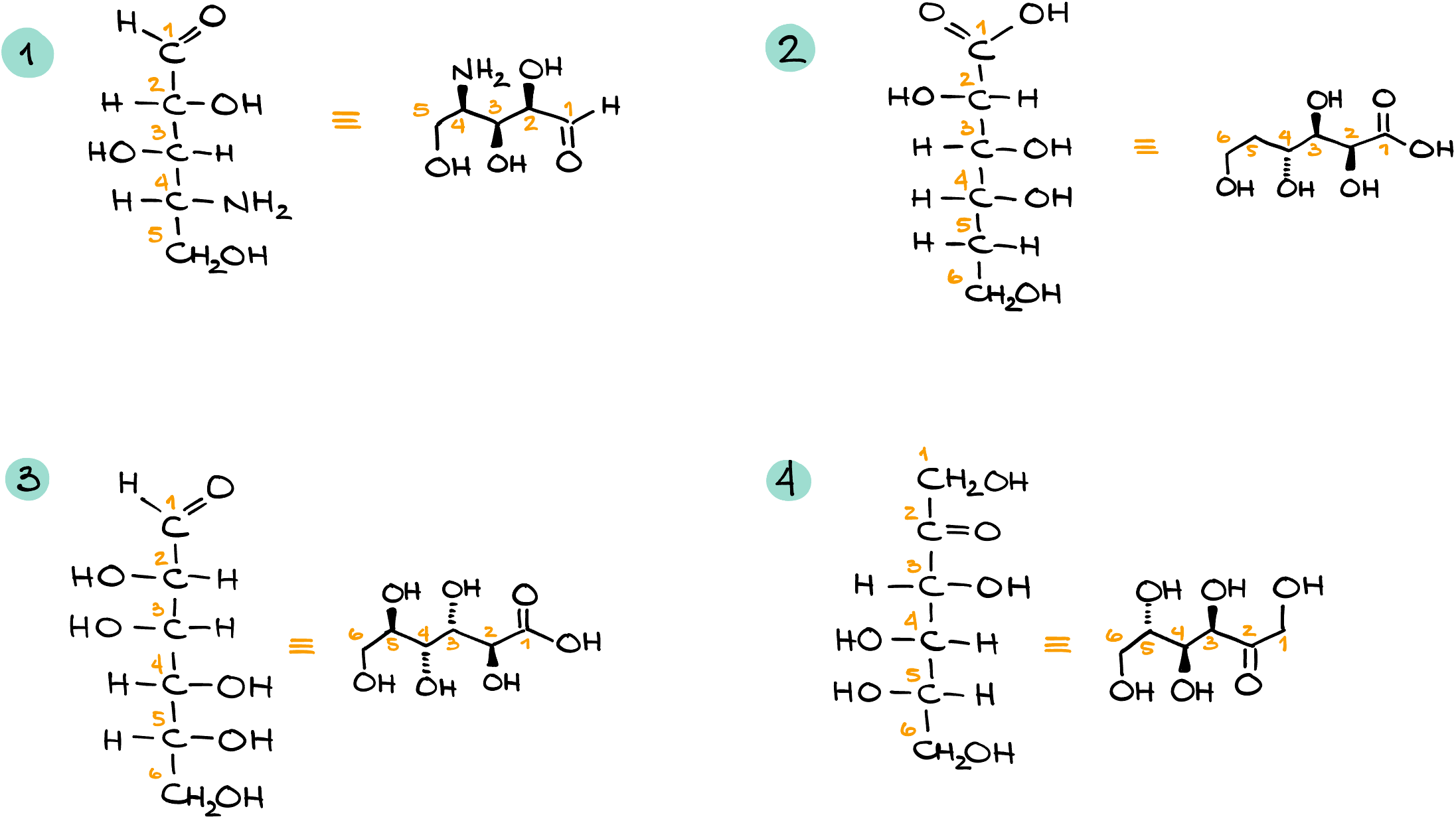
Glucose (a carbohydrate):
The simple straight-line representation we often see for glucose can be visualized as a 3D molecule. When viewed from the side, those straight lines transform into the familiar dashed and wedged bonds.
Alanine (an amino acid):
Just like with glucose, the linear Fischer projection of alanine corresponds to a more dimensional view when you look at it from a different angle.
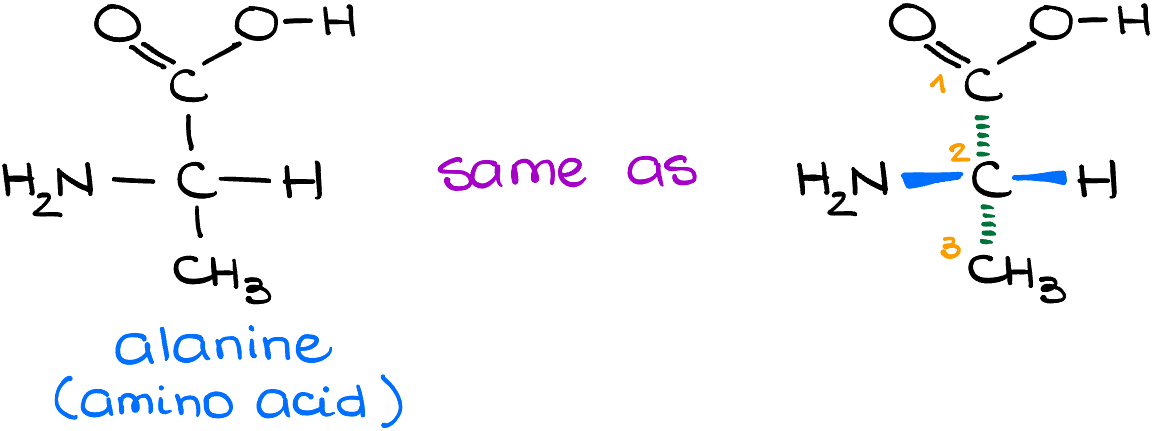
Why is this Important?
Fischer Projections are essential tools, especially when dealing with molecules that have multiple chiral centers. They provide a clear and concise way to represent the spatial arrangement of atoms.
A Bit of History:
Fischer Projections were introduced by the German chemist Emil Fischer in the late 19th century. Fischer’s work was groundbreaking. He was able to represent complex 3D structures on paper, revolutionizing the way chemists visualized and communicated molecular structures.
How to Convert Fischer Projections int Dash-and-Wedge Bond-Line Structures
We’ve previously looked at Fischer Projections, but how do we translate these 2D drawings to more familiar bond-line structures? Let’s unravel this mystery together.
The Conversion Process:
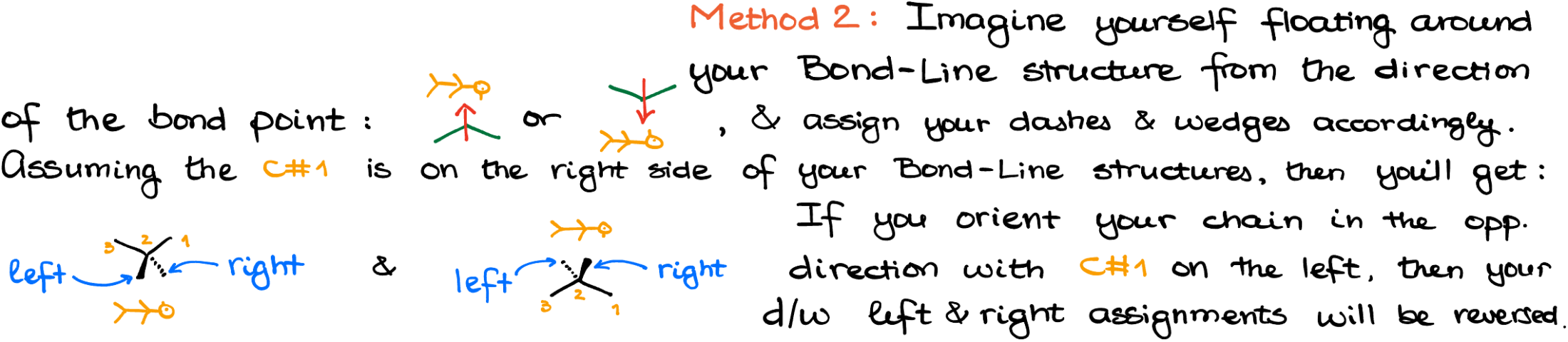
When converting a Fischer projection to a bond-line structure, you’re essentially visualizing the molecule in 3D. The process can be approached in multiple ways.
Method 1: Assign the R/S stereodescriptors and then do the same for the bond-line structures. If you guess correctly, move to the next atom, if not, just switch the dashes and wedges.

Method 2: Imagine yourself floating around the molecule. This one requires a bit more visual prowess, but with some practice this method gives you super quick results!
Examples