Conjugated Systems and Pericyclic Reactions
A conjugated system is a type of a molecule where you have multiple p-orbitals interacting with each other. Generally, you’ll need 3 or more orbitals to classify a molecule as conjugated. Thus, the simplest example of a conjugated system is an allylic ion or a similar molecule with 3 adjacent atoms each with a p-orbital.
Examples:
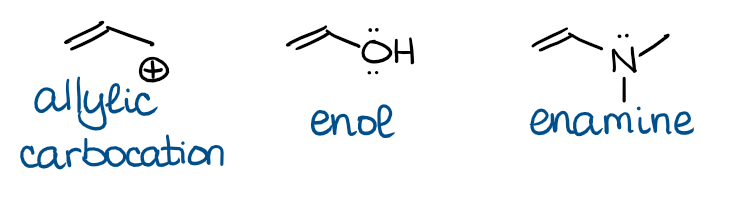
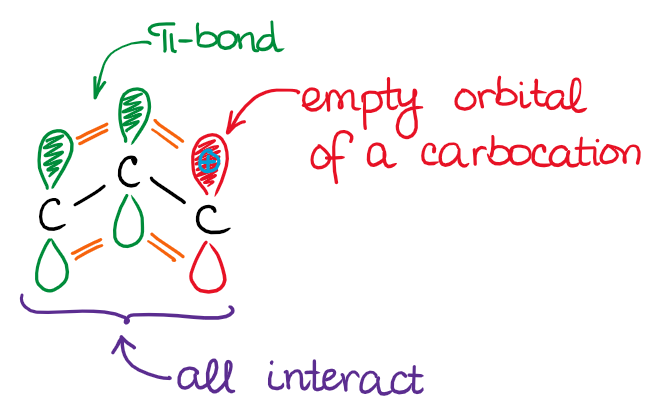
In these molecules and ions, the π-bond interacts with either an empty orbital (allylic carbocation) or an electron pair on a heteroatom (enol and enamine). The important thing about the conjugated systems, is that all interacting orbitals must be in the same plane to interact. Here’s how the orbitals in an allylic system look like:
Essentially, anything you can write resonance structures for and has 3 atoms or more, can be classified as a conjugated system. In this lesson and the subsequent few we’ll focus on the Molecular Orbitals of conjugated systems and their reactions.
