Acetal Mechanism Challenge
Here, we have a rather complex looking lactone (cyclic ester) reacting with an acid. And as a result we get a much simpler lactone. Also, by quickly looking at our carbons here, I can see that we’ve lost a few carbons, which means that something fell off our molecule in the process.
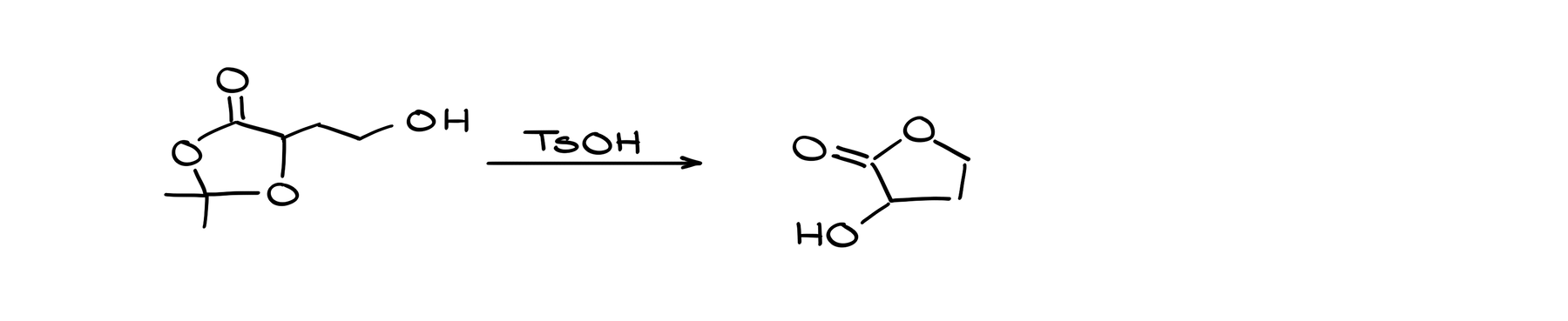
Now, looking at this reaction, my first instinct would be to analyze where exactly our initial protonation is going to happen. The thing is, there are quite a few different places here that can be protonated. So, how do we choose?
Typically, when I’m analyzing my molecules while trying to come up with a mechanism, I aim to look a couple of steps ahead. Kind of like in chess, where you’re trying to predict the board state a few moves ahead. And the best position here is going to be the protonation of my carbonyl of the ester functional group.
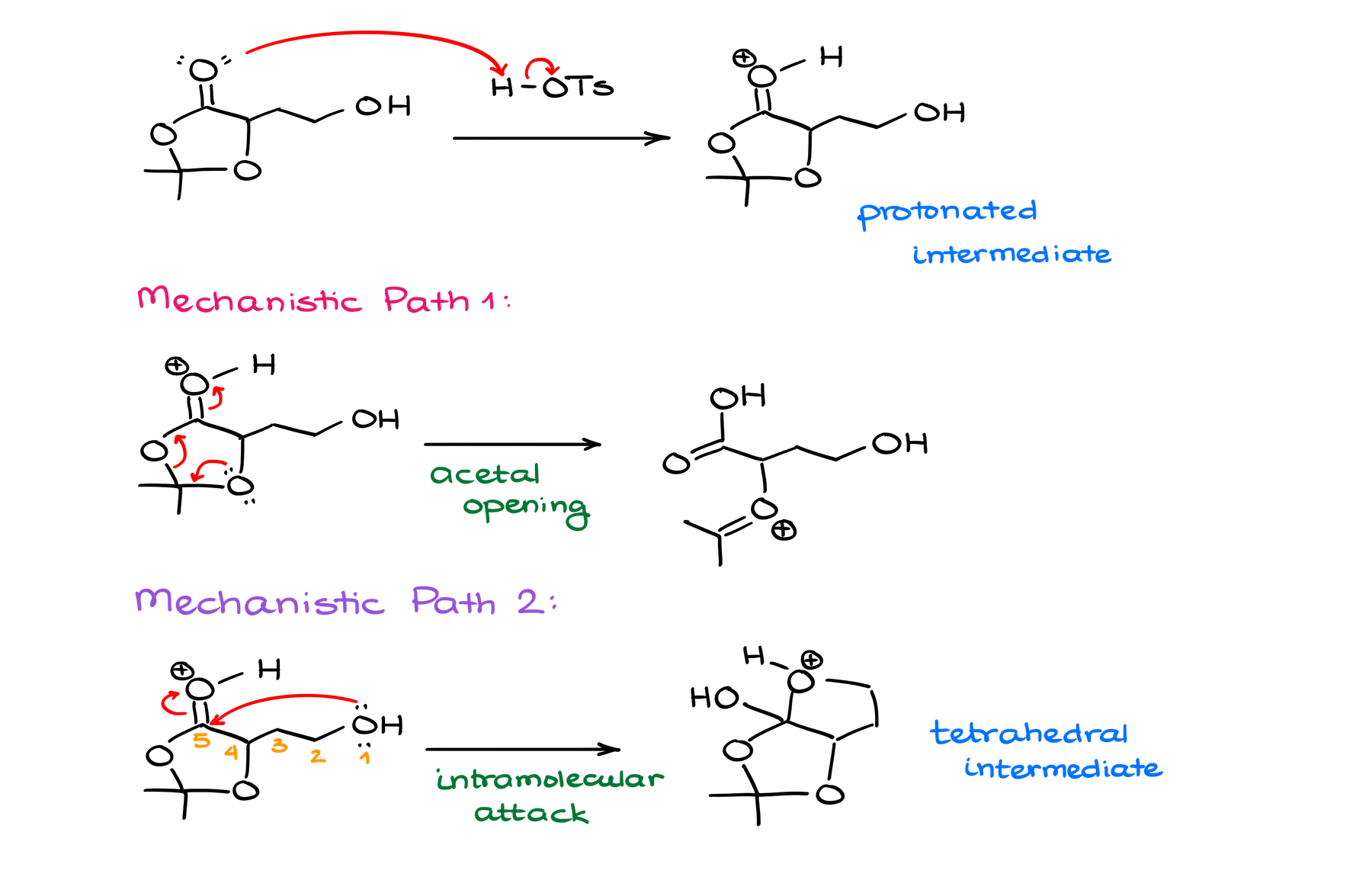
From that point, we have a couple of possible pathways for our mechanism. The first would be to break up our acetal. And the other one would be to do the intramolecular attack on the ester, essentially, starting an intramolecular Fischer trans-esterification reaction.
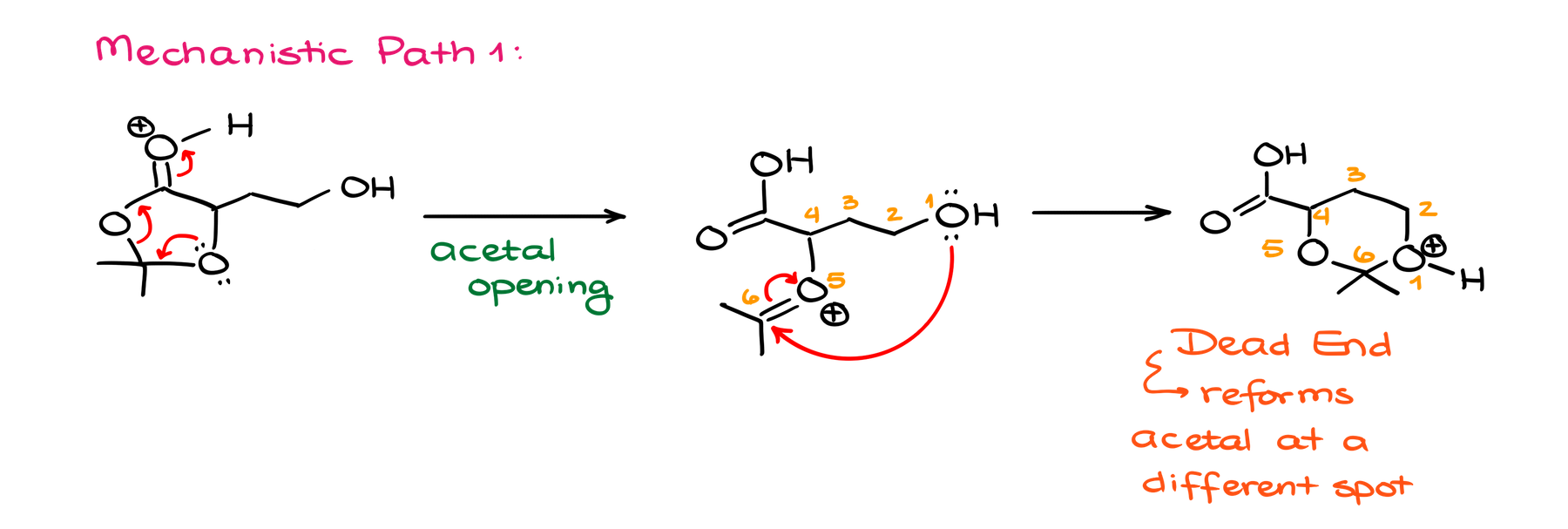
The former one is a dead end as this would take me to a species that can potentially make another acetal, but then what? We’re back to square one, and we didn’t progress our reaction towards our final product. If anything, we took it in a completely different direction!
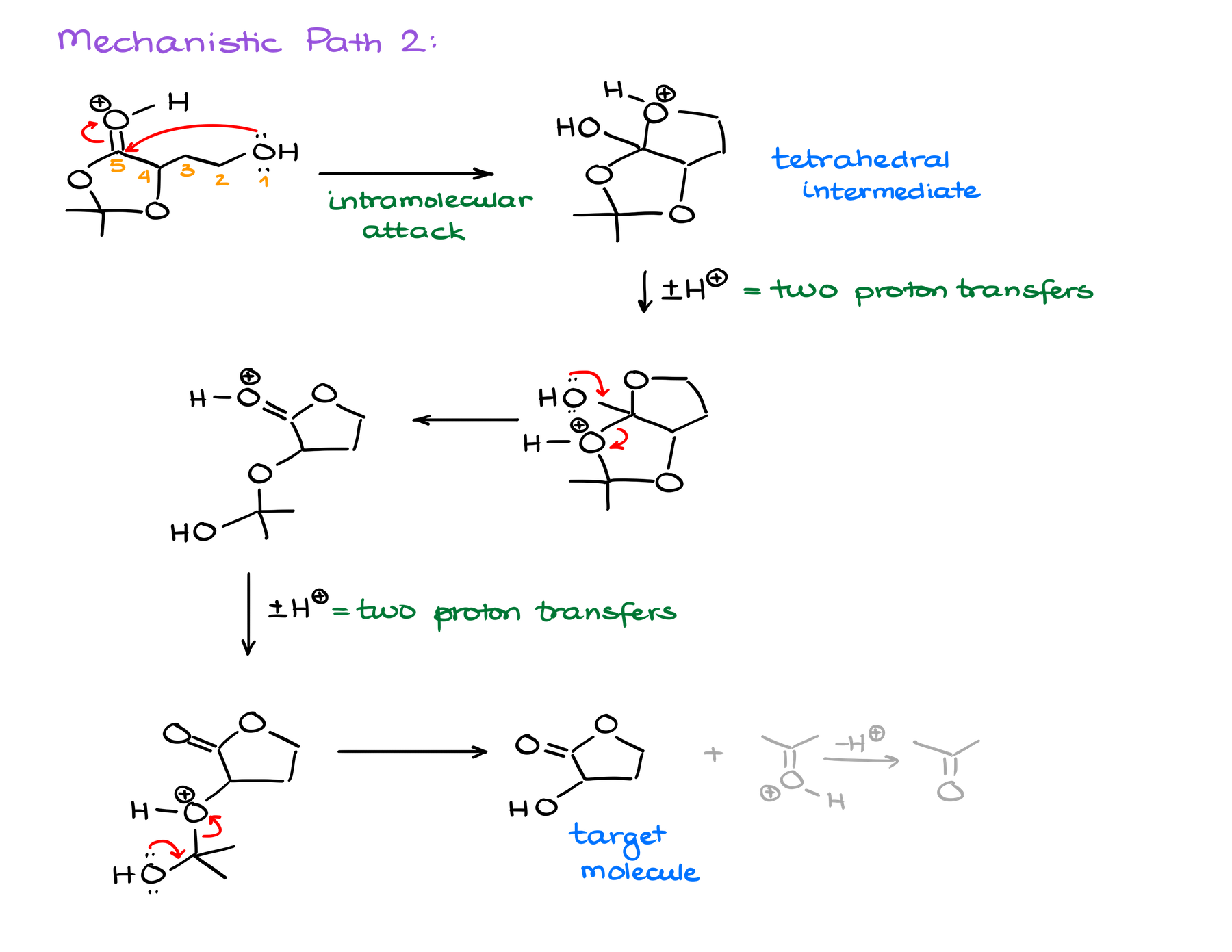
The latter option, however, looks promising. Once I’m done with the trans-esterification steps, I end up with a hemiacetal functional group. And we know that those are quite unstable and tend to decompose giving you the corresponding carbonyl and an alcohol. Well, if something like that would happen here, we would end up with the target molecule and acetone molecule as a co-product!
So, when you’re working on mechanisms like this one, it might be tempting to just protonate something and go with it and see what happens. And while this approach might get you somewhere, it’s likely to either get you to a dead end, make your mechanism take a strange turn, or take you to a completely incorrect final product. So, what I suggest is try a step or two and brainstorm where it’s taking you. If you see that your mechanism is taking a wrong turn, try something different. There’s no shame in going back and tracing your steps and rethinking a part of the mechanism. With more practice, you’ll start seeing chunks of the mechanism few steps ahead. But you need to build that skill first. And until then, brainstorm mercilessly and never go with the first option and brute force your way through the mechanism! Brute forcing your mechanism and forcing the formation of your product regardless of what’s reasonable is the absolute worst you can do! And unfortunately, I see student fall into that trap all the time.
