How to Estimate the pKa Values Using the pKa Table
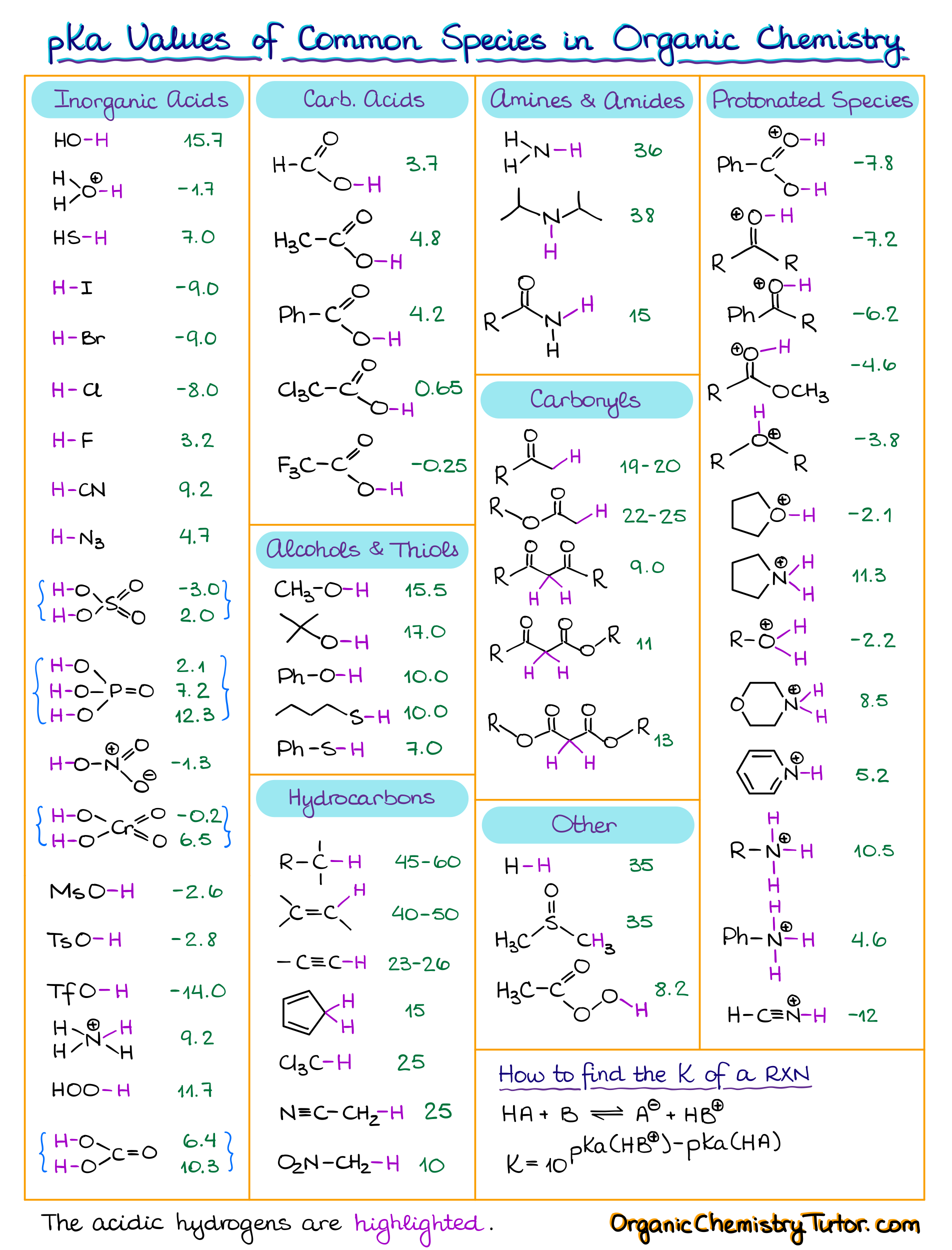
In the realm of organic chemistry, understanding acid-base reactions is paramount. It forms the foundation for comprehending a myriad of chemical processes. To navigate this complex field, chemists often turn to pKa tables as indispensable tools. In this article, we will delve into the intricacies of pKa tables, discussing how to use them effectively to solve acid-base-related questions. These tables are widely utilized in organic chemistry, helping chemists estimate the strength of acids and bases. However, the key lies in understanding how to interpret and apply the information they provide.
Understanding pKa Tables
Types of pKa Tables
Before diving into the specifics of pKa tables, it’s essential to recognize that there are different types of these tables. You may encounter them in various formats, depending on your course materials, instructor, or textbook. Some instructors provide their own preferred pKa tables to ensure consistency in their teaching materials. These tables can be organized in two primary ways: by functional groups or by pKa values.
- Functional Group Organization: In this approach, the pKa values are grouped based on the functional groups they belong to. For instance, all carboxylic acids may be listed together, followed by alcohols, amines, and so on. This organization makes it easier to identify the pKa of a specific functional group in a molecule.
- pKa Value Organization: Alternatively, pKa tables can be organized based on the actual pKa values, either in ascending or descending order. For instance, the table may start with the lowest pKa values and progress to the highest or vice versa. This arrangement allows chemists to quickly locate the molecule with the pKa value they need.
The Limitations of pKa Tables
One crucial aspect to grasp is that no pKa table is exhaustive. It won’t contain every compound you might encounter in your organic chemistry journey. Consequently, the challenge lies in knowing how to use the information provided in the table to estimate pKa values for compounds not explicitly listed. In most cases, you won’t find the exact pKa value for the compound you’re working with. This tutorial aims to guide you on how to make educated estimations based on the available data.
Estimating pKa Values: A Step-by-Step Guide
Estimating pKa values for compounds not listed in the table is a skill that every organic chemist and student taking organic chemistry should develop. Here’s a step-by-step approach to help you make accurate estimations:
- Identify the Functional Group: Begin by identifying the functional group(s) in the molecule for which you need to estimate the pKa value. Understanding the functional groups is crucial since pKa values are largely determined by the type of functional group present.
- Analyze Hybridization: Consider the hybridization state of the atoms within the functional group. This detail can offer valuable insights into the acidity or basicity of the compound.
- Check for Charge: Determine if there are any charges within the molecule. Positive and negative charges can significantly affect the pKa value. Positive charges suggest an acidic compound, while negative charges imply a basic one.
- Search the Table for Similar Features: Look for compounds in the pKa table that share similar features with the one you’re working with. These features could include the type of functional group, hybridization state, and charge.
- Estimate the pKa Value: Once you’ve found a compound in the table that closely resembles the one you’re investigating, take note of its pKa value. This value will serve as your estimation.
- Compare with Experimental Data: If possible, compare your estimated pKa value with experimental data. While it may not match precisely, a reasonably close estimate is generally acceptable for most organic chemistry applications.
Case Studies: Estimating pKa Values
To illustrate this process further, let’s explore a few case studies:
- Amine Group: If you encounter a molecule with a nitrogen atom connected to an sp3 hybridized atom and a positive charge, it likely represents a protonated amine. In the pKa table, find a similar protonated amine, such as CH3NH3+, with a pKa value of 10.6.
- Alcohol Group: For a molecule with an oxygen atom in an sp3 hybridized state connected to another sp3 hybridized carbon, you’re dealing with an alcohol. Estimate its pKa to be around 16, given that primary alcohols typically have pKa values near 16.
- Carboxylic Acid: Recognize a carboxylic acid by the presence of the carboxyl functional group. Estimate its pKa value to be approximately 4.8, as carboxylic acids typically fall within the 4 to 5 range.
- Protonated Pyridine: When you encounter a molecule resembling protonated pyridine, with sp2 hybridized nitrogen in an aromatic ring and a positive charge, find a similar compound in the pKa table. Protonated pyridine has a pKa value of 5.2.
- Unusual Compound with Fluorine Atoms: In cases where you encounter a compound with highly electronegative atoms like fluorine near the acidic hydrogen, make a conservative estimate. In this instance, the closest match is trichloroacetic acid with a pKa of 0.64, but the actual value may be even lower, around 0.2.
- Sulfur-Hydrogen Bond: Identify a sulfur-hydrogen bond, then search for a similar compound in the pKa table. For example, ethyl thiol has an estimated pKa of 10.5, which is reasonably close to the experimental value of around 9.5 for the compound under investigation.
- Complex Molecule with Carbonyls: Analyzing complex molecules may require a more in-depth approach. For instance, when dealing with a compound with hydrogen situated between two carbonyl groups, estimate its pKa value by finding a similar compound in the pKa table, such as acetylacetone with an estimated pKa of 9.2.
Conclusion
In conclusion, pKa tables are indispensable tools for understanding acid-base chemistry in organic chemistry. While they may not contain every compound you encounter, they provide valuable information to estimate pKa values for a wide range of compounds. By focusing on functional groups, hybridization, charge, and similar features, you can make informed estimations that are generally suitable for your studies and research. Remember that your instructor may provide a specific pKa table, so familiarize yourself with it to ensure consistency in your coursework. Ultimately, mastering the use of pKa tables is a crucial skill for any organic chemist, and it opens the door to a deeper understanding of the fascinating world of acid-base reactions.
