Lewis Acids and Bases
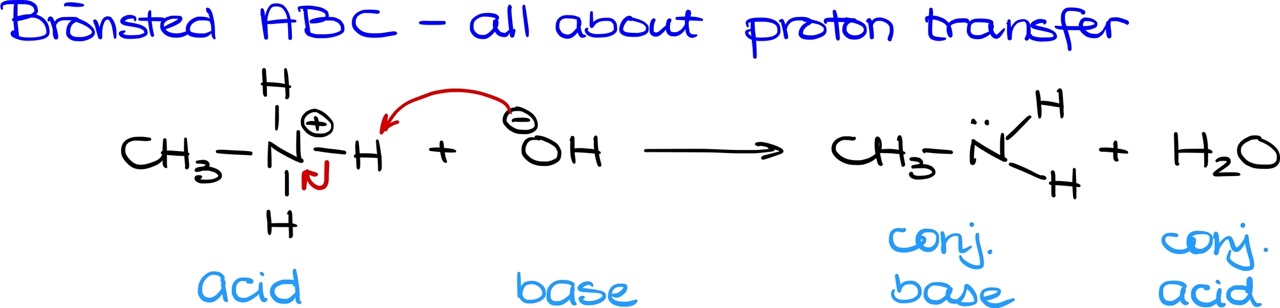
In organic chemistry, we often emphasize the Bronsted-Lowry theory, which centers around proton transfer. Let’s look at an example I’ve got up here. The proton transfer occurs between the OH and our protonated amine. What’s happening is that the OH is taking a proton, and as a result, our water turns into its conjugate acid. With Bronsted-Lowry, we discuss acids and bases in pairs: the original acid or base and its corresponding conjugate.
On the other hand, the Lewis theory focuses on electron sharing, leading to the formation of complexes instead of proton transfers. In this theory, a base forms a new bond with an acid, creating a complex between the two elements.
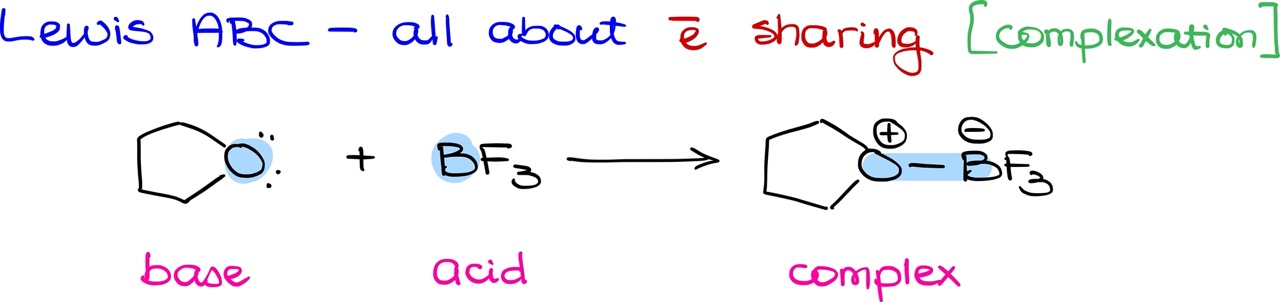
What are the Lewis Acids and Bases?
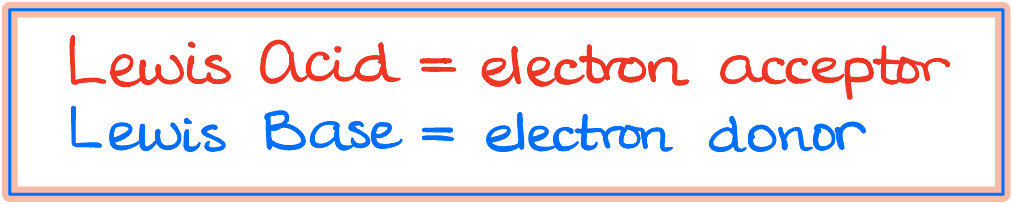
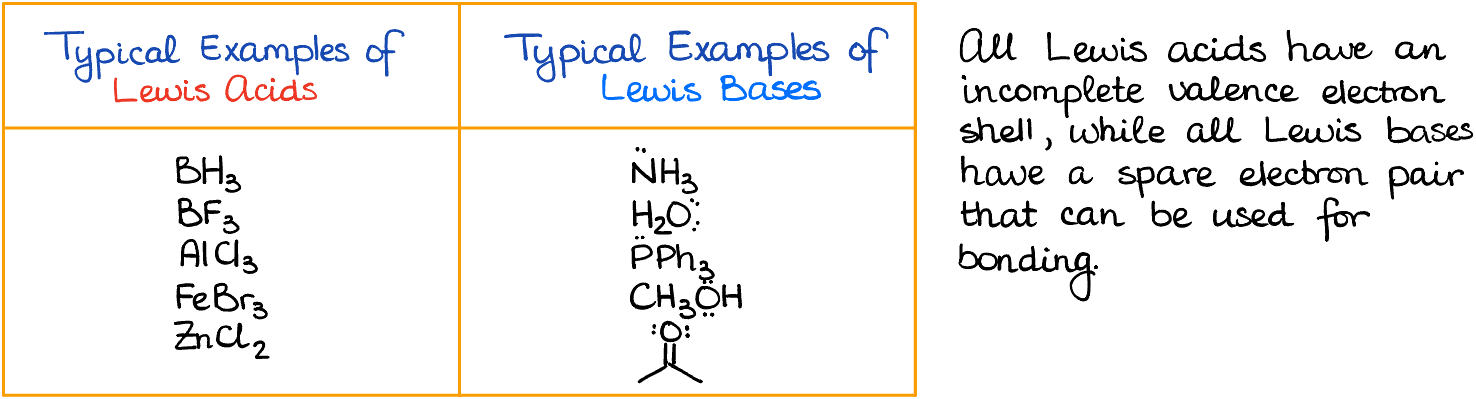
When delving deeper into Lewis acids and bases, it’s essential to understand their fundamental characteristics. A Lewis acid acts as an electron acceptor. Typically, any molecule or ion with an empty orbital ready to accept electrons is a Lewis acid. Some examples include:
- H+: A proton devoid of electrons.
- Carbocations, like CH3+.
- Boron compounds, such as BH3 or BF3, both of which have an empty orbital. You can think of these orbitals as ‘little boxes’ waiting for electrons.
- Metal-containing compounds, like AlCl3 (aluminum chloride), FeBr3 (iron bromide), and TiCl4 (titanium chloride). These metals, with potential empty D orbitals, are ready to take in electrons.
On the flip side, a Lewis base donates electron density. Essentially, any molecule with a free electron pair qualifies. For instance:
- Water (H2O): It has electron pairs, making it a suitable Lewis base.
- OH- anion: It boasts three electron pairs.
- Ammonia (NH3): This has an electron pair ready for donation.
- Alcohols: They have electron pairs.
- Chloride anion (Cl-): It comes equipped with four electron pairs.
- Triphenylphosphine: It too has an electron pair, making it a viable Lewis base.
Examples of the Lewis Acid-Base Reactions
In the Lewis acid-base complexation reaction, these two entities (an acid and a base) come together, resulting in a more substantial molecule.
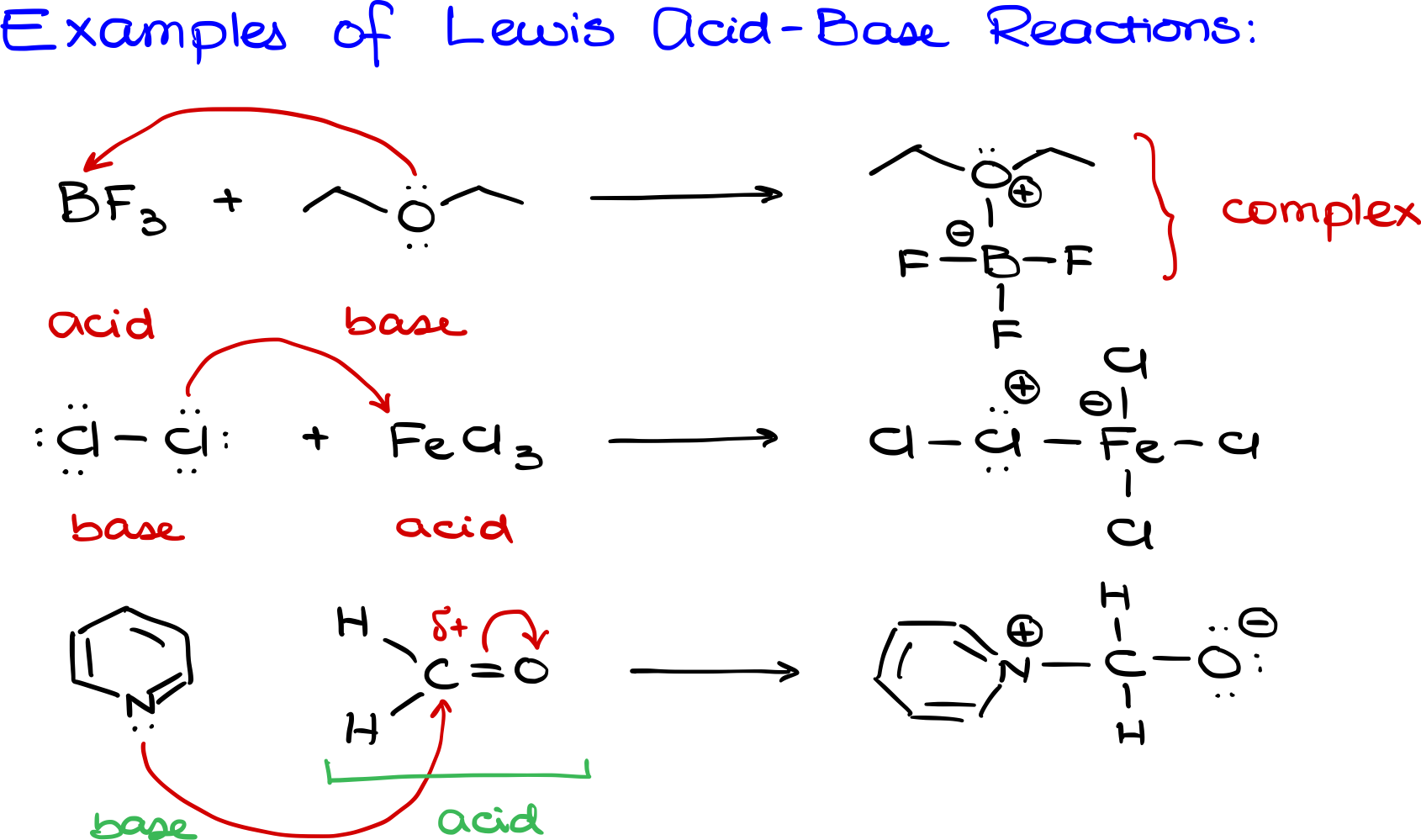
So for instance let me illustrate it on the example I have BF3 which is a good Lewis acid because it can accept electrons and I have let’s say a diethyl ether molecule over here which can donate electron density and serve as a base. In this case oxygen is going to give electron density to the boron and what we are going to end up with as a result is a new boron oxygen bond where I have my boron still surrounded by all of my fluorines, but now because oxygen gave the electron density to make a new bond with boron we’re going to have a formal plus on the oxygen and because boron has accepted the electron density we’re going to assign the formal minus to our boron. In this case my acid is going to be boron trifluoride so I’m going to say that this is my acid and my base is going to be the species that gave the electron density to make a bond thus it is my diethyl ether. The resulting species that I have over here is going to be my complex and it doesn’t have any other fancy name or anything of that sort, they’re just some sort of a complex where two molecules came together to make one bigger molecule.Here’s how the reaction unfolds:
In this scenario:
- Acid: Boron trifluoride (BF3)
- Base: Diethyl ether, because it willingly offered its electron density to form the bond.
- Product: This resulting entity doesn’t bear a fancy name. Simply put, it’s a ‘complex.’ Essentially, two distinct molecules have joined forces to create a larger, unified molecule.”
Let’s look at another example of a Lewis acid-base reaction: Imagine a chlorine molecule – a simple diatomic chlorine (Cl2) – reacting with iron chloride (FeCl3) pictured above.
Iron in FeCl3 has an empty orbital eager to welcome more electrons. When presented with chlorine, the latter generously donates its electrons to iron. This act classifies chlorine as the Lewis base and iron as the Lewis acid.
The outcome? A new bond forms between chlorine and iron. While illustrating this bond, it’s crucial not to forget the other chlorines previously surrounding the iron. Since chlorine offered up its electron density to form this new bond, it bears a formal positive charge. In contrast, iron, having received this electron generosity, carries a formal negative charge.
Many Different Reactions Can be Classified as Lewis Acid-Base Equilibria
Sometimes, reactions not typically categorized under the Lewis acid-base umbrella still get classified as such. Let’s take the reaction between a pyridine molecule and formaldehyde as an example (figure above).
Pyridine can donate electron density to the carbon in formaldehyde. Although this carbon doesn’t have a vacant orbital ready to accept electrons, it does have a significant positive charge, making it electrophilic and hungry for extra electron density. To accommodate the electrons from pyridine, carbon pushes some of its electron density onto the oxygen via the pi bond.
The result? A bond forms between the nitrogen in pyridine and the carbon in formaldehyde. This transition sees oxygen taking on a formal negative charge, since it receives the pushed electrons. Meanwhile, nitrogen, the initial electron donor, ends up with a formal positive charge.
Though this might not seem like your typical Lewis acid-base reaction, some educators prefer this classification over the nucleophile-electrophile distinction. Here’s a tip: Whenever a reaction involves one entity donating electron density to another to form a bond, you can possibly label it as a Lewis acid-base reaction. In our case, nitrogen (from pyridine) donates, making it the base, while carbon (from formaldehyde) accepts, deeming it the acid.
Stability of the Lewis Complexes
While we’re not going to be assessing the Keq for the Lewis acid-base reactions, we can assess the comparative stability of different complexes depending on what atoms make a new bond.
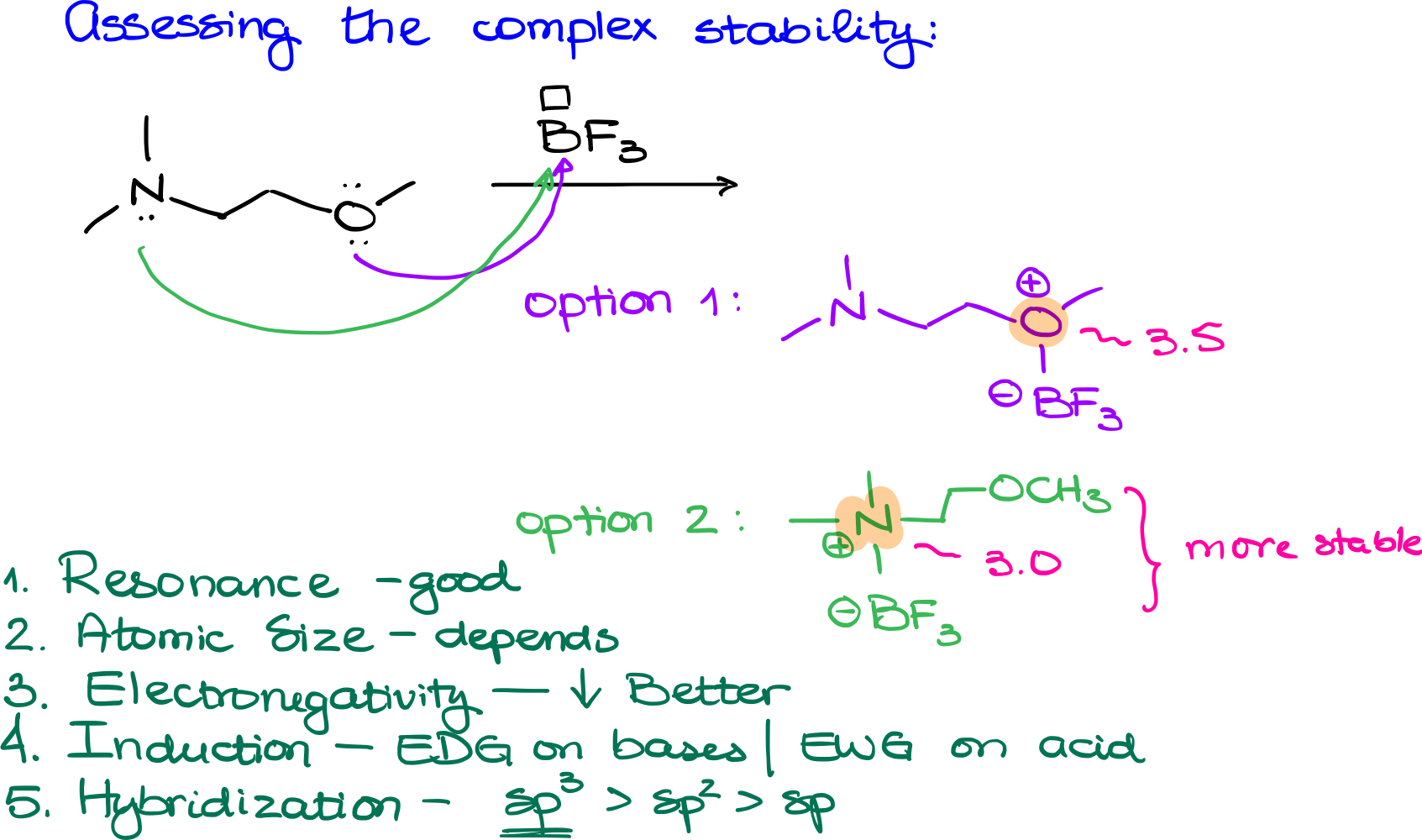
Let’s look at the example above, nitrogen and oxygen-containing compounds can act as our Lewis bases due to the presence of electron pairs on both nitrogen and oxygen. With boron having an empty orbital, it serves as the Lewis acid. Given the two potential reaction sites, either on the nitrogen or the oxygen, we can anticipate two possible product outcomes:
- Oxygen Bonds with Boron: This results in one molecular structure.
- Nitrogen Bonds with Boron: This leads to a different molecular structure.
To determine which complex is more stable, I’ll rely on the same five factors used for Brønsted-Lowry acid-base reactions:
- Resonance: This stabilizes charges, making molecules with resonating charges generally more stable.
- Atomic Size: The better the orbital overlap between species, the more stable the bond. Atoms of similar sizes, like oxygen and boron or nitrogen and boron, bond well because of their good orbital overlap. However, attempting a bond between two drastically different-sized elements creates a ‘frustrated Lewis pair’, which isn’t very stable.
- Electronegativity: In many Lewis acid-base complexes, a positive charge is formed. The stability of this charge is greater when it’s on a less electronegative atom.
- Induction: Electron-donating groups enhance the “basicity” of our Lewis bases. In contrast, electron-withdrawing groups boost the reactivity of acids, making them more eager to accept electrons.
- Hybridization: The ideal Lewis base has an electron pair on an sp3-hybridized atom, which is slightly more reactive than those on sp2, and much more than sp.
Using these criteria, you can evaluate the stability of various Lewis acid-base complexes.
When examining our two complexes, the charge is situated on the oxygen in the first and on the nitrogen in the second. Let’s analyze their stability using our established criteria:
- Resonance: Both molecules exhibit no distinguishable differences in terms of resonance.
- Atomic Size: Oxygen, nitrogen, and boron belong to the same periodic period, so their atomic sizes are comparable.
- Electronegativity: This is where the difference emerges. Oxygen has an electronegativity of about 3.5, while nitrogen’s is closer to 3.0. A positive charge is more stable on nitrogen due to its lower electronegativity compared to oxygen.
Based on our analysis, the complex where nitrogen holds the charge (our second option) is more stable. So, in a laboratory setting, the second option would likely be the predominant product.
Using the pKa Values as a “Proxy” to Assess the Stability of a Lewis Complex
A handy trick involves using the pKa table to gauge the stability of our complexes. Let’s revisit the example from earlier. Instead of reacting the starting material with a Lewis acid, we’ll use H+ as a stand-in. Even though H+ also acts as a Lewis acid, it differs from the one initially used. This gives rise to two potential products. In the first scenario, where nitrogen is protonated, the product will have a positive charge on the nitrogen. On the other hand, if oxygen is protonated, the positive charge will reside on the oxygen.
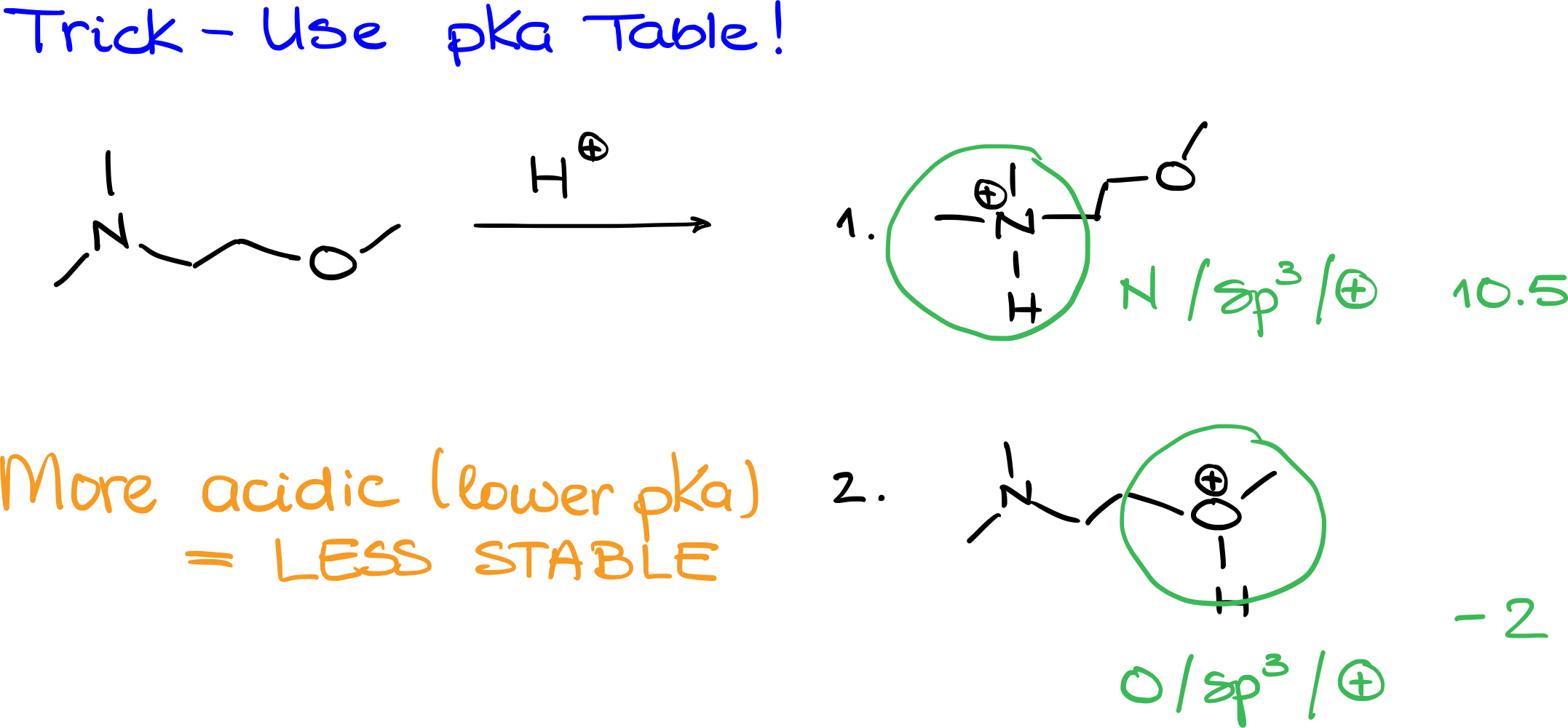
To determine which of these species is more stable, we can reference the pKa table. For the species where nitrogen is protonated, its characteristics (sp3-hybridized with a positive charge) suggest a pKa value of around 10.5 to 10.6. For the species with the protonated oxygen, it aligns with properties of a protonated ether or alcohol, leading to a pKa value near -2. This indicates that the protonated oxygen species is much more acidic and hence, wants to relinquish its proton more eagerly than the nitrogen variant. Consequently, the more acidic protonated oxygen species is less stable.
It’s important to note that this approach isn’t universally applicable to all Lewis acid-base reactions. However, when dealing with species that have parallels in the pKa table, this method can offer valuable insights into relative stabilities.
In organic chemistry, while our primary focus isn’t on Lewis acid-base reactions, it’s crucial to be able to identify and understand them. Even though they may not frequently come up, there are times when recognizing Lewis acids and bases can be essential.
