Nomenclature of Alkenes and Cycloalkenes
Understanding Alkenes
Alkenes are hydrocarbons that contain a carbon-carbon double bond, represented by C=C. This double bond is a defining feature of alkenes and plays a crucial role in their reactivity and properties.
Step-by-Step Guide to Naming Alkenes
Identifying the Primary Chain
- Start by selecting the longest carbon chain that contains the C=C double bond. This becomes your primary chain.
- In the case of multiple double bonds, ensure the primary chain contains the most C=C bonds.
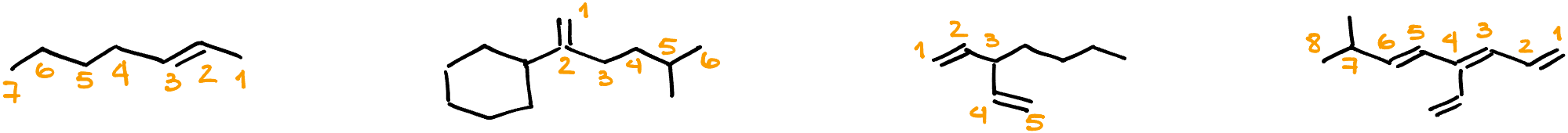
Numbering the Chain
- Begin numbering from the end closest to the first C=C.
- For cyclic compounds, choose the carbon of the C=C that leads to the shortest route to the next substituent or double bond. Fun Fact: The numbering system in organic nomenclature was designed for clarity and consistency, ensuring scientists across the globe speak the same ‘chemical language’.

Breaking Ties in Numbering
- If you find multiple numbering options, two tiebreakers come to your rescue:
- First, opt for the sequence that gives the lowest numbers to substituents.
- If a tie persists, proceed alphabetically.
Composing the Name
- Start with the names of substituents in alphabetical order.
- Indicate the position of the C=C by using numbers.
- End with the suffix “-ene” for alkenes.
- For multiple double bonds, use “-diene”, “-triene”, and so on, depending on the number of double bonds.
Examples to Illuminate the Concept:
Compound with Bromine and Chlorine Substituents:
- Name: 6-Bromo-5-chlorohex-1-ene
- Here, the numbering starts from the end closest to the C=C, and substituents are placed in alphabetical order.

Compound with Multiple Substituents and Double Bonds:
- Name: 5-chloro-3-ethyl-6-methylhepta-1,4-diene
- In this case, there are two C=C bonds, leading to the “-diene” suffix.
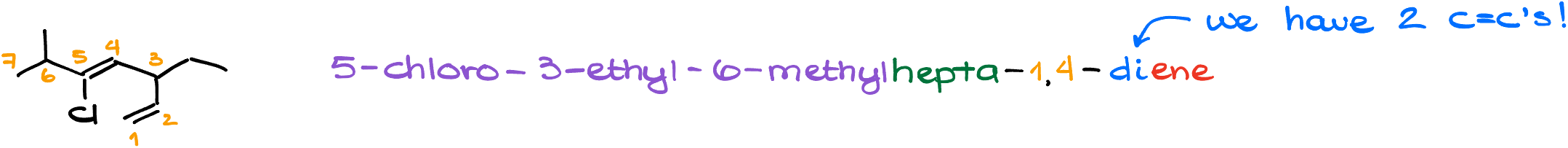
Cyclic Compound with Substituents:
- Name: 1-chloro-3-ethyl-3,5-dimethylcyclohexene
- Cyclic compounds are named similarly, but the cycle’s name becomes part of the base name.
In Summary:
Naming alkenes can be both fun and straightforward! Just remember to identify the primary chain, number it appropriately, and use the correct suffix. With practice, you’ll become an alkene naming expert in no time. Remember, organic chemistry is a journey, and every step brings a new discovery. Keep exploring!
