Acidity of Carbonyls
Here are some typical pKa values describing the acidities of common carbonyls that we usually see in organic chemistry. If we look at those numbers, they are kind of all over the place.
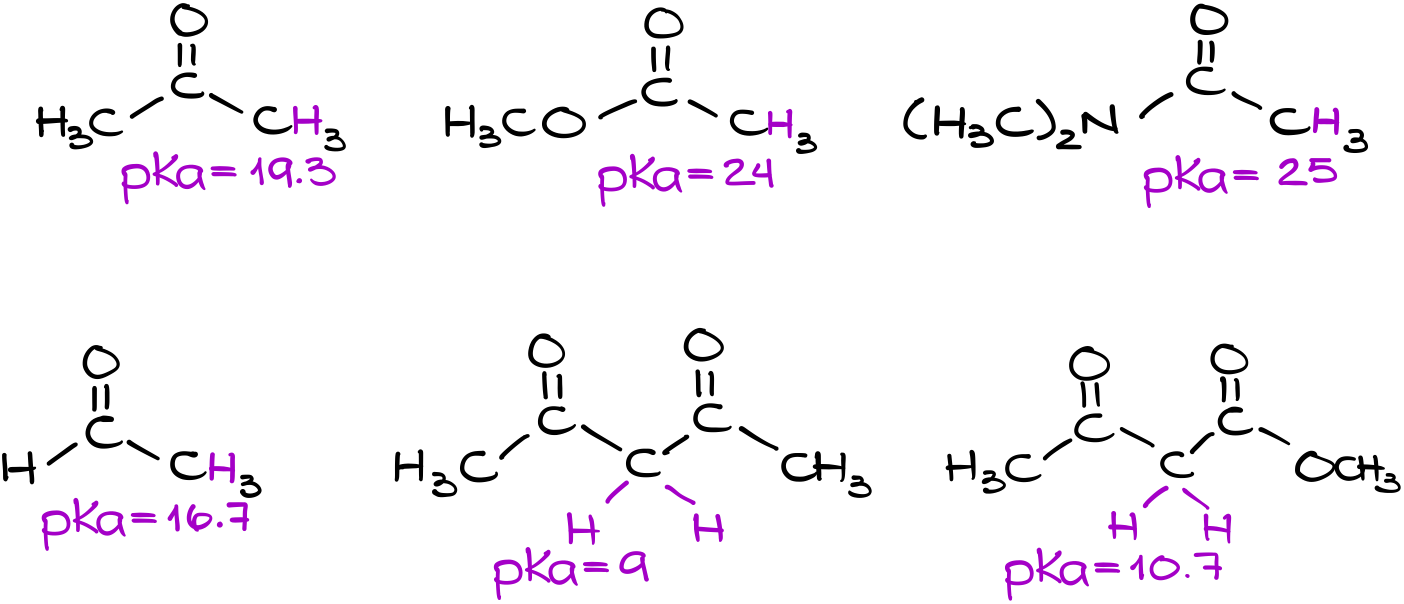
We’ve seen over and over again that a typical reaction of carbonyls is either a nucleophilic addition to aldehydes and ketones, or various acyl substitutions of carboxylic acid derivatives. However, the adjacent position to the carbonyl (the ⍺-position) exhibits a lot of interesting chemical properties as well. The most important of these properties is the significantly higher acidity of the ⍺-protons compared to the typical alkanes.
If we pull out a pKa table, we’ll see that the pKa values for the ⍺-position in carbonyls ranges from anywhere between 9 and mid 20’s. So, what might be the cause for such a wide range?
Stability of the Enolate Species
Let’s look at a simple deprotonation reaction, in which we’ll take an acetone molecule and react it with a base. The nature of the base here is not relevant.
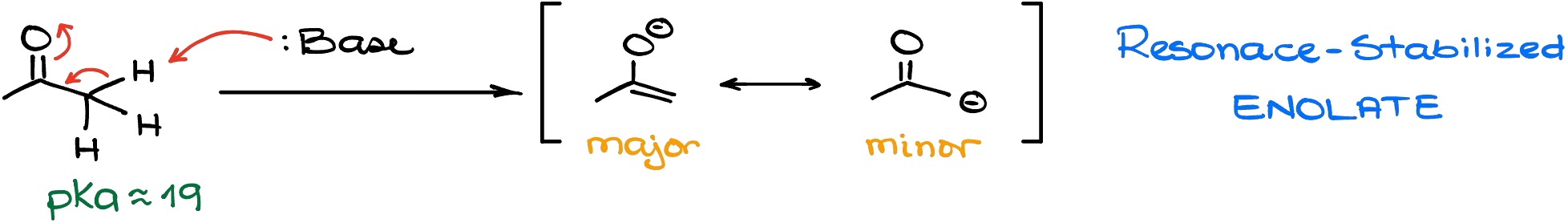
As the result, we’re going to get a negatively charged species which we call and “enolate” anion or simply enolate for short. This enolate is stabilized by resonance. However, we only have one major resonance contributor with the negative charge on the oxygen atom. The other contributor – the one with the negative charge on the carbon atom – is the minor contributor and doesn’t contribute much towards the stability of the anionic species we have here. Because of that, the pKa value that we see for the ⍺-position in acetone is about 19. Not as high as for a regular alkane (~55), but not quite low to be considered acidic either.
However, if we look at a carbonyl compound with two C=O bonds near to each other, we’ll have a strikingly different situation:
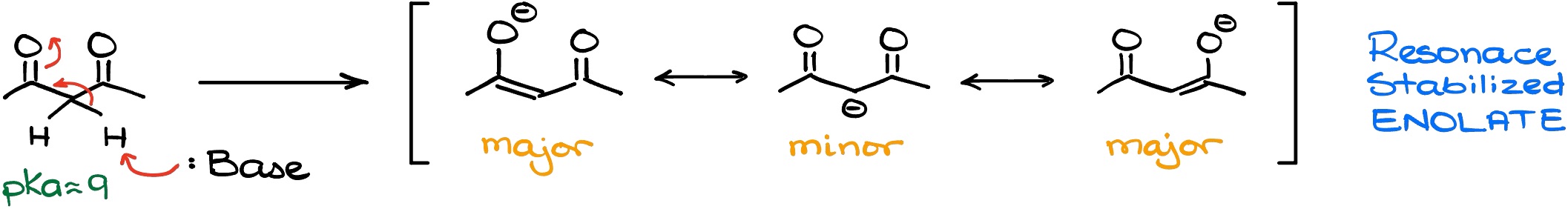
Now, we have the enolate which is stabilized by two major resonance contributors. And as we have seen in many cases before, the more major resonance contributors we have, the more stable the species is. This brings our pKa value down to 9! Which is 1010 times more acidic than what we saw in the previous example!
Resonance vs Induction
We just saw how the resonance affects the acidity of the carbonyls. But there are 5 factors that we typically consider in the acid-base equilibrium. And another big factor is the induction. If we have Electron-Withdrawing Groups (EWG) or electronegative elements close to the location of our anion, we typically see increased acidity and lower pKa values. Are we going to see the same here? Well, yes and no. Let’s look at a couple of examples here.
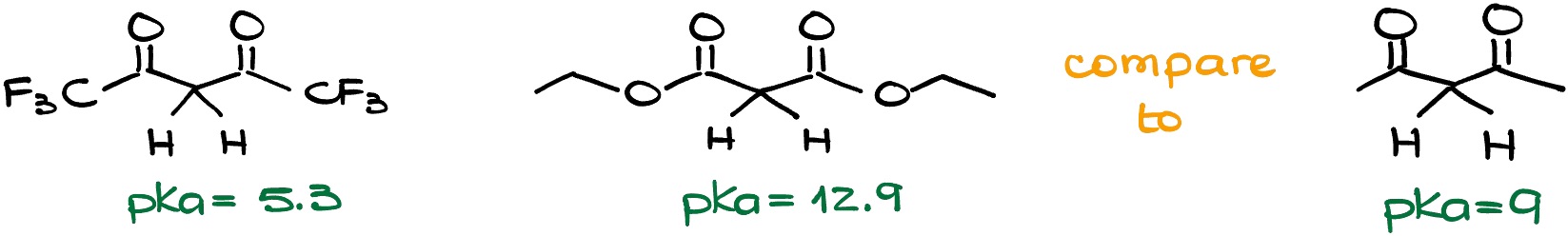
If we have a trifluoromethyl group and our EWG, then we indeed see the drop in the pKa values which means that our molecule is more acidic. But if we have an ethoxy group, the pKa value didn’t only not drop, but it increased, which means our molecule is less acidic! What’s going on here?
The trick here is that in the first case, we have a pure inductive effect. Fluorine atoms pull electron density towards themselves and give nothing back. However, in the second case, we have a competition between the inductive effect and the resonance effect! Oxygens pull the electron density towards themselves via the inductive effect. But at the same time, they donate the electron density towards the carbonyl via the resonance effect.
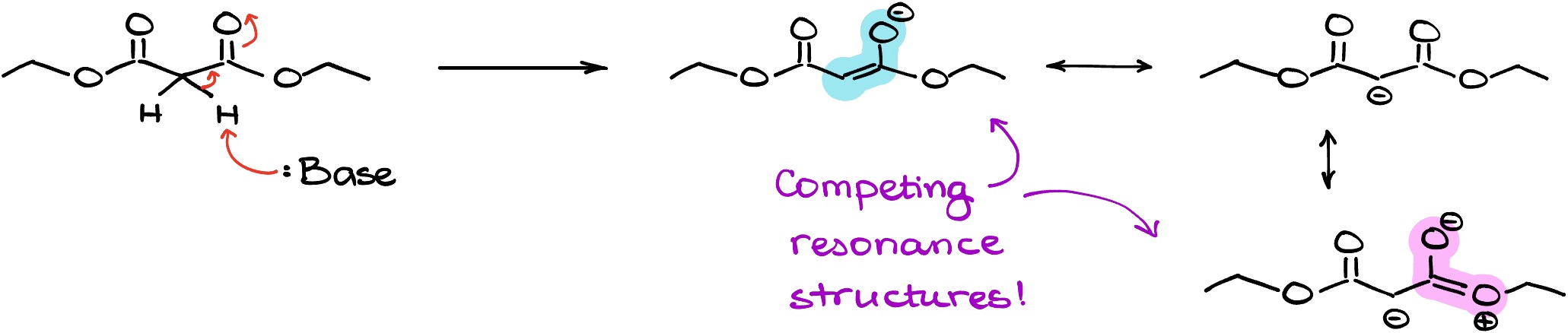
And here’s the problem we have with that. Since we need a carbonyl to stabilize the negative charge on the carbon, we don’t really want something else to disrupt our resonance delocalization. But that’s precisely what the oxygen of the ethoxy group is doing. It’s not adding to the continuous resonance stabilization, it’s pulling our resonance in a different direction. And if our carbonyl is going to be constantly pulled into either resonance with the oxygen or the resonance with the negative charge, it’s not going to be effectively participating in either. So, we’re going to see a significant decrease in the resonance stabilization of the anion from the carboxylic acid derivatives.
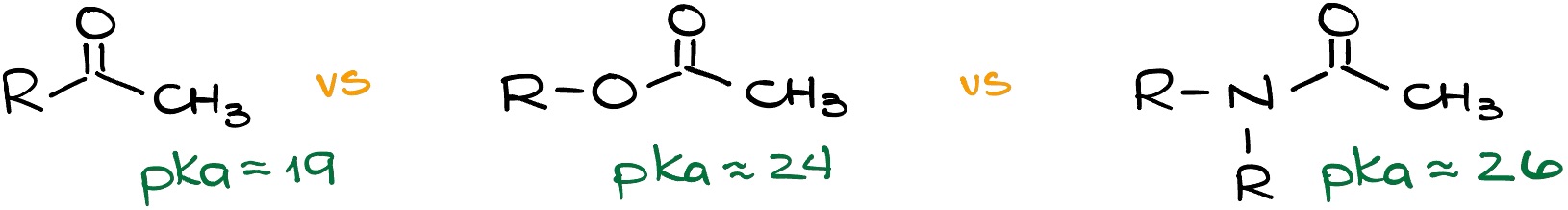
And importantly, the stronger the resonance in the carboxylic acid derivative, the weaker the stabilization effect we’re going to see for the enolate. Thus, esters are less acidic than ketones, and amides are less acidic than esters.
Know Your pKa Values!
So, since there is quite a spread of the pKa values among different carbonyls, it’s going to be very important for you to actually know those numbers or at least know the ballpark of those. You may wonder as to why? Just take a base that would be strong enough to yeet that proton, and you’re all set! Well, the problem with that approach is that we’re always going to pull off the most acidic proton first. Let me illustrate with an example:
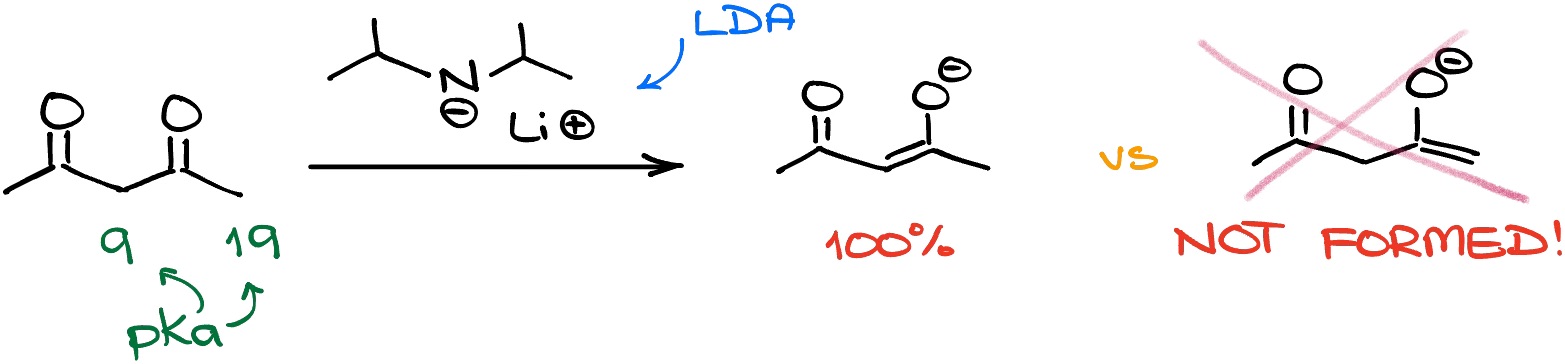
In the molecule above, I have two places where we can pull the proton from. One, one the end of the molecule, has the pKa value around 19. The other one – between the carbonyls – has the pKa around 9. This is a huge difference! And it doesn’t matter which base you use in this case. In my example I used LDA on purpose because that base is strong enough to pull off either of those protons. And yet, we still see the more acidic come off first.
You might argue that this was an extreme example. But trust me, you’ll see many more examples in your course, where smaller differences are going to be equally as important and will sway your reaction towards one final product or another. So, if you don’t pay attention to the pKa values, you can easily walk into a trap on the test.
Does that mean that you have to memorize the numbers? Also no. While I do encourage you to memorize the numbers eventually, don’t just sit down and learnt the list by heart. This would likely be a very frustrating experience and will make a mess in your head. Instead, do a lot of practice and you’ll remember the numbers as you work through the problems. For now, just make a quick cheat sheet with the typical pKa values you’re going to see for the carbonyls and keep it handy.
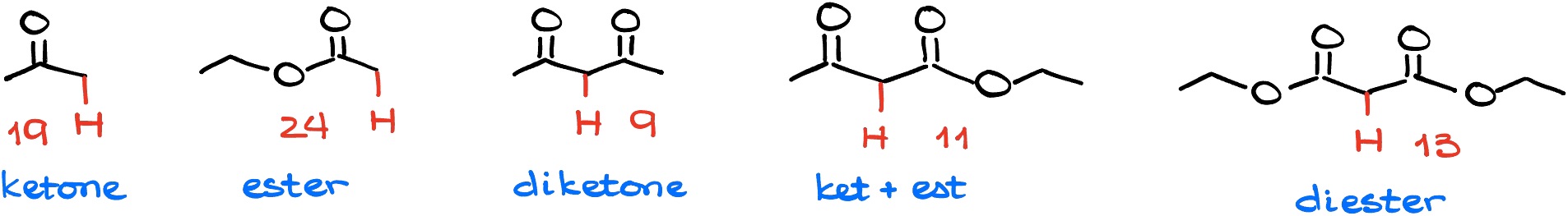
Above, is the list I tell my students to memorize. As you can see, it’s just 5 values! Pretty easy to remember!
