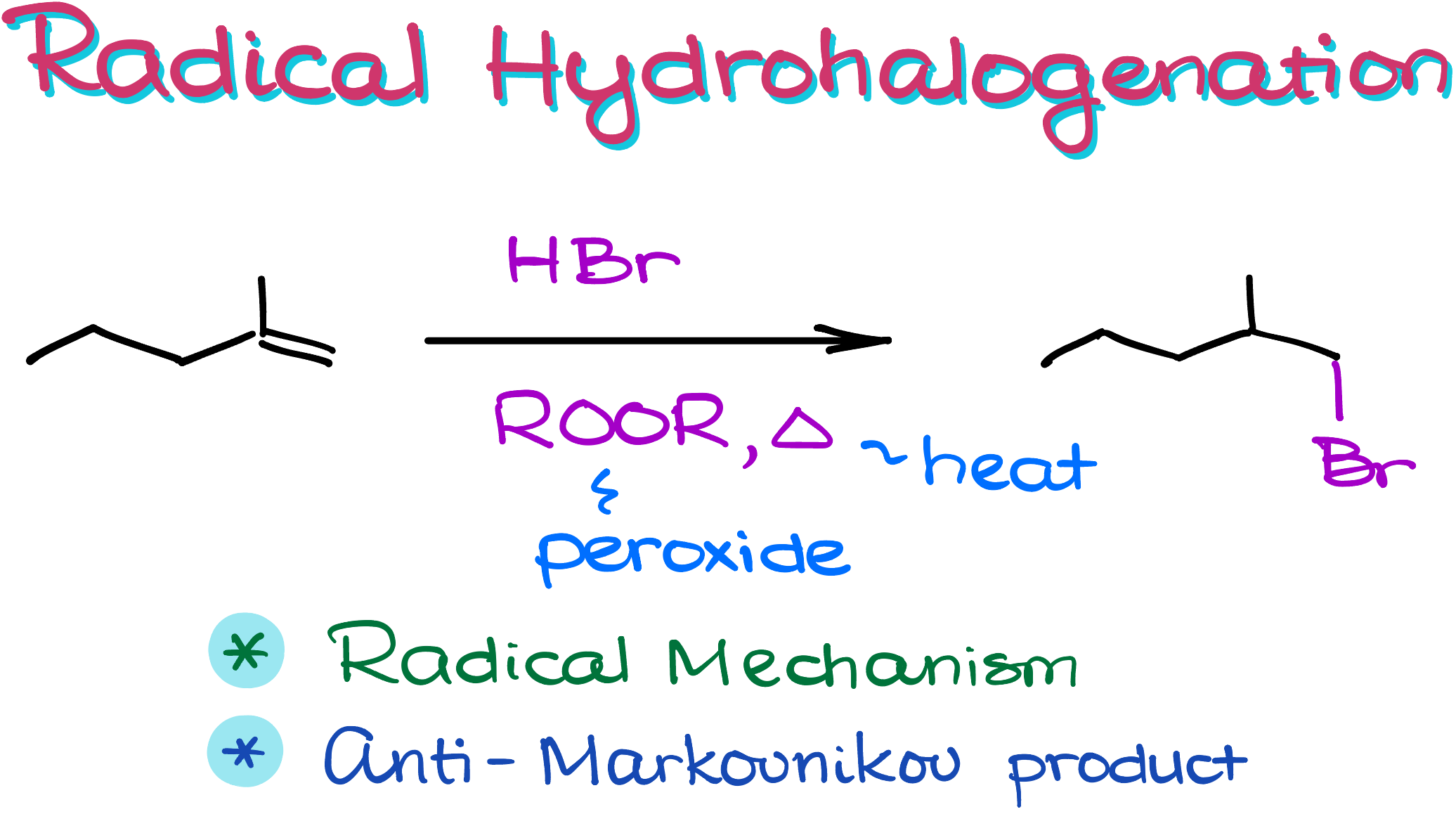
Radical Hydrohalogenation of Alkenes (Anti-Markovnikov Addition of HBr to Alkenes)
In this tutorial we’ll look at something very different from our typical mechanisms. Here we’ll talk about radical hydrohalogenation. This is one of the few reactions that gives us an anti-Markovnikov product. This makes radical hydrohalogenation an important synthetic procedure which you’ll most likely have to use on your organic chemistry final when dealing with the multistep synthesis.
Review of Radicals
So, first of all, what exactly is a radical? A radical is a neutral—or in other words—uncharged species, bearing a single unpaired electron.
And since radicals operate with single electrons, we’re also going to be using different kind of arrows to show the electron movement. We’re going to be using the so-called “fishhook” or half-arrows. Notably, since we’re going to be showing the movement of each single electron, the number of arrows you’ll see in radical reaction mechanisms is always going to be quite large.
Mechanism of the Radical Hydrohalogenation
As with any radical reaction, we’re going to start with the initiation step. Here’s where the organic peroxide is going to break into two initial radicals.
Initiation
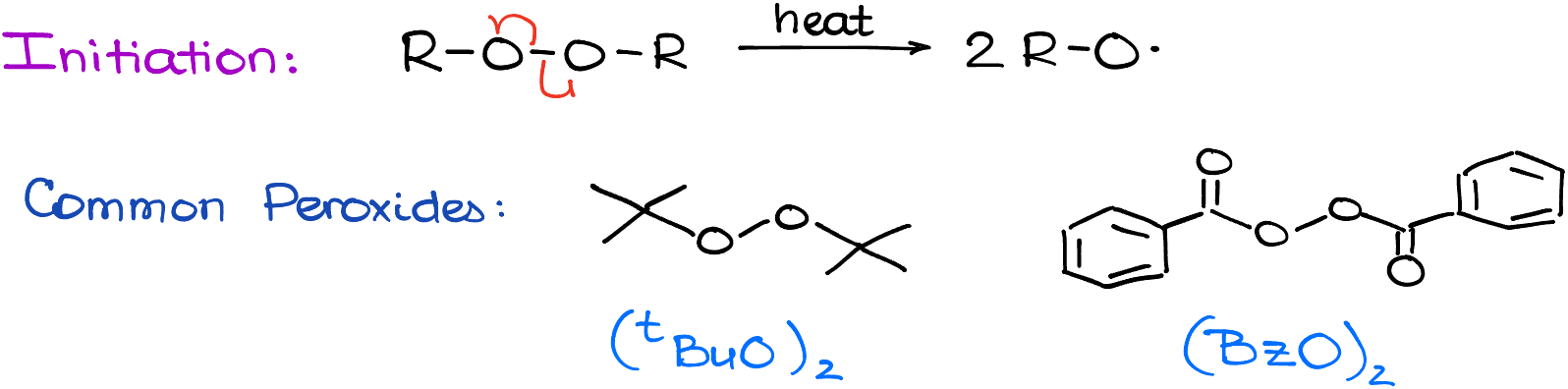
The most common peroxides used in the lab are the tert-butyl peroxide and benzoyl peroxide. There are others, of course, but in my experience, these two are the most common. Sometimes, you can see that some instructors and even some textbooks use the hydrogen peroxide, which is a bit of a white lie, as H2O2 doesn’t break into radicals as easily as they make it seem.
For the simplicity’s sake, I’ll just use ROOR here. If your instructor likes a specific peroxide, well, use that one 🤷♂️.

Next, our newly formed peroxide is going to scavenge a hydrogen from HBr. This step gives us the Br-radical, which will actually kick-start our propagation cycle.
Propagation
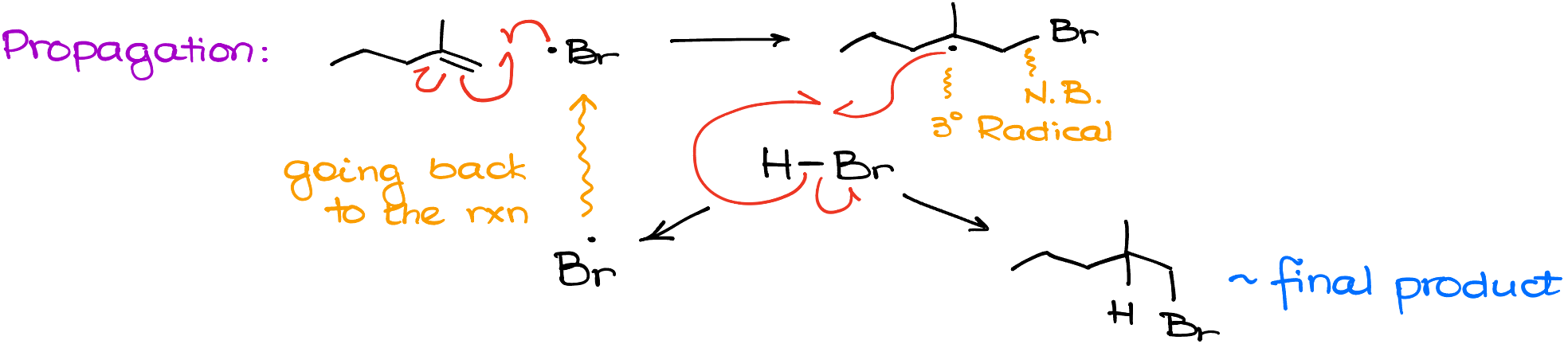
So, the first step of the propagation is going to be the interaction between the Br-radical and the alkene.
In this reaction, one electron will come from bromine. Next, another electron comes from the pi-bond to make a bond between bromine and carbon. And finally, the second electron from the pi-bond stays with another carbon of what used to be a double bond. This gives us the product in which we get the new bond between carbon and bromine, and we’ll also see a tertiary radical.
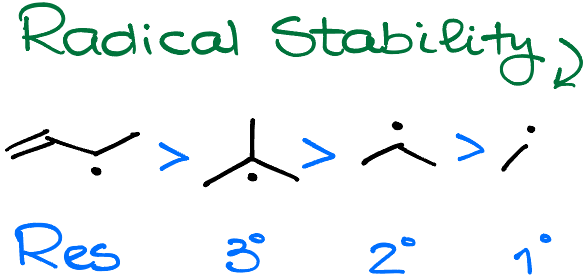
These reactions will always give you the most stable radical. And when it comes to the radical stability, they follow the same trend as carbocations: resonance stabilization beats the 3° radical, which is more stable than the 2° radical, which in turn, is more stable than the 1° radical.
Next, our organic radical will grab a hydrogen from another molecule of HBr.
This regenerates the Br-radical that can go back into another round of the propagation cycle, and the final product.
Termination
And, of course, like in any radical reaction, we’ll also need a termination step that terminates the propagation cycle.

Here, I’ll only show one of the many possible termination steps. If you wanna practice, I suggest you write the termination steps for all possible combinations of radicals in this reaction. Theoretically speaking, any two radicals that exist in this mechanism (Br-radical, alkyl radical, alkoxide radical) can randomly find each other in space and recombine. We can argue all day long which is more likely and which recombination is less likely, but overall, the products of the termination step will always make a negligible quantity of the final reaction mixture, so who cares.
Mechanism Overview
So, to recap, we start with an initiation step in which we form our radicals. This is where we’ll break up our peroxide, typically with a little bit of heat.
Next, the alkoxide radical will find the HBr in the mixture and interact with that. This yields a Br-radical which we’re going to send into the propagation cycle.
In the propagation cycle, we first have the Br-radical interact with the alkene making the alkyl radical. What’s important to remember here, is that we’re always going to make a more stable radical out of two possible ones.
Then, the alkyl radical going to grab the hydrogen off the HBr molecule giving us the final product and the Br-radical, which goes to another cycle of the propagation process restarting it all over again.
Examples of Radical Hydrohalogenation to Alkenes
Alright, let’s look at a few examples.
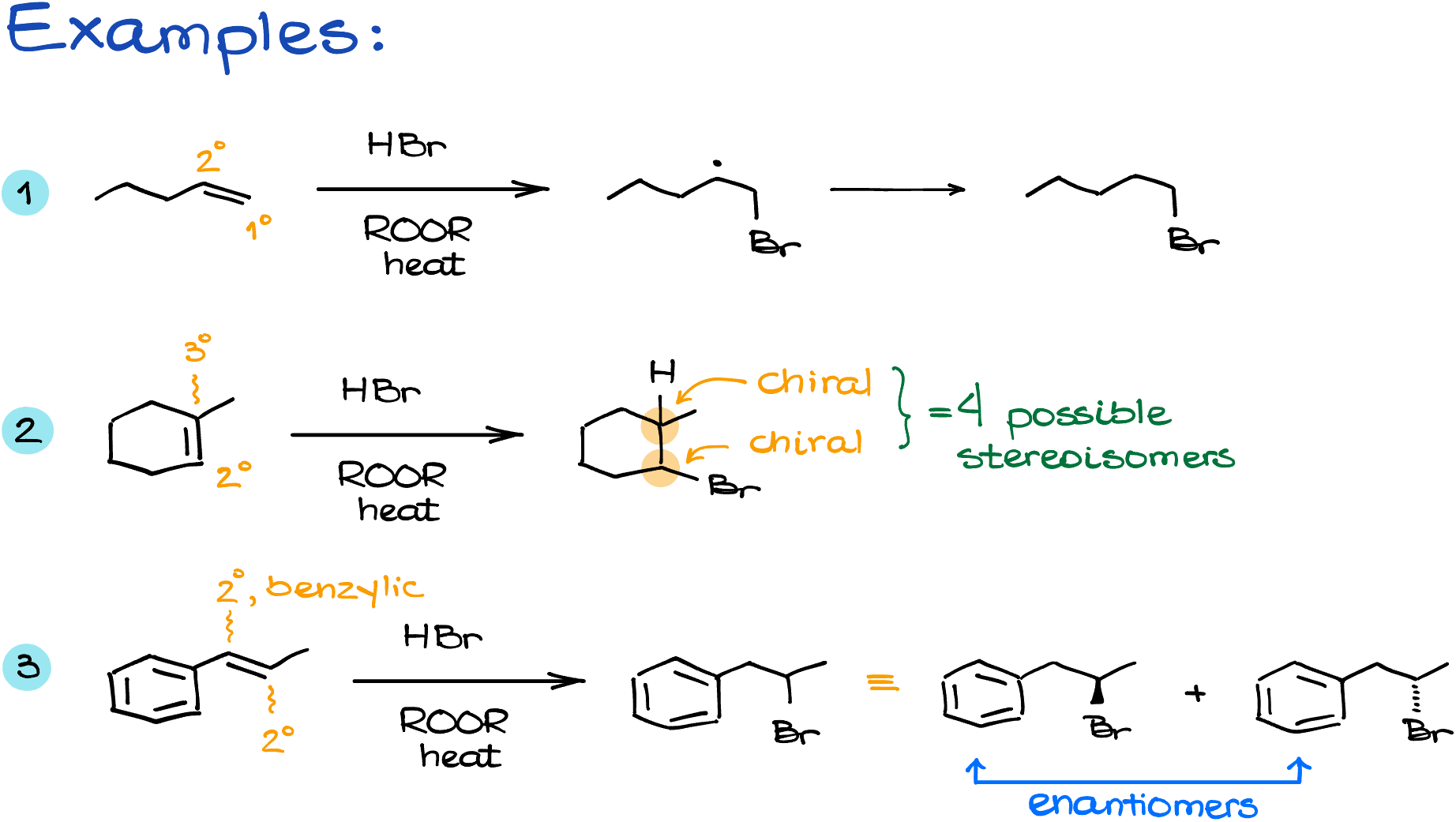
Recap
Alright, to recap, remember that the radical hydrohalogenation is a radical process and uses its own special rules for the electron movements using the fishhook or half-arrow.
We need a radical initiator to kick-start this reaction. Typically, we’re going to use organic peroxides.
We’re always going to form the most stable radical in this reaction. So, you should always prioritize the resonance stabilized and 3° radicals before 2° and 1°.
Because of that, this reaction leads to the product where you’re going to see the bromine attached to the less substituted atom. Or in other words, this reaction is said to give you the anti-Markovnikov product.
And finally, this reaction is not stereospecific. So, it leads to the formation of all possible stereoisomers if you’re creating any chiral carbons. So, be careful with this one, as some instructors require student to draw all stereoisomers if you’re making several of those guys.
