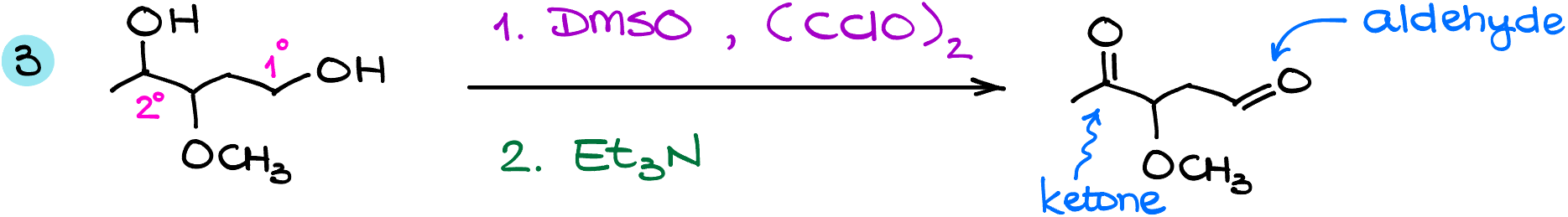Swern Oxidation
The Swern oxidation is one of the most commonly taught alternatives to the PCC. Is the Swern oxidation an ideal alternative? Certainly not. This reaction is incredibly stinky and requires some harsh reagents. But, it is much safer than the PCC since we don’t have to deal with the chromium containing compounds which are quite toxic.
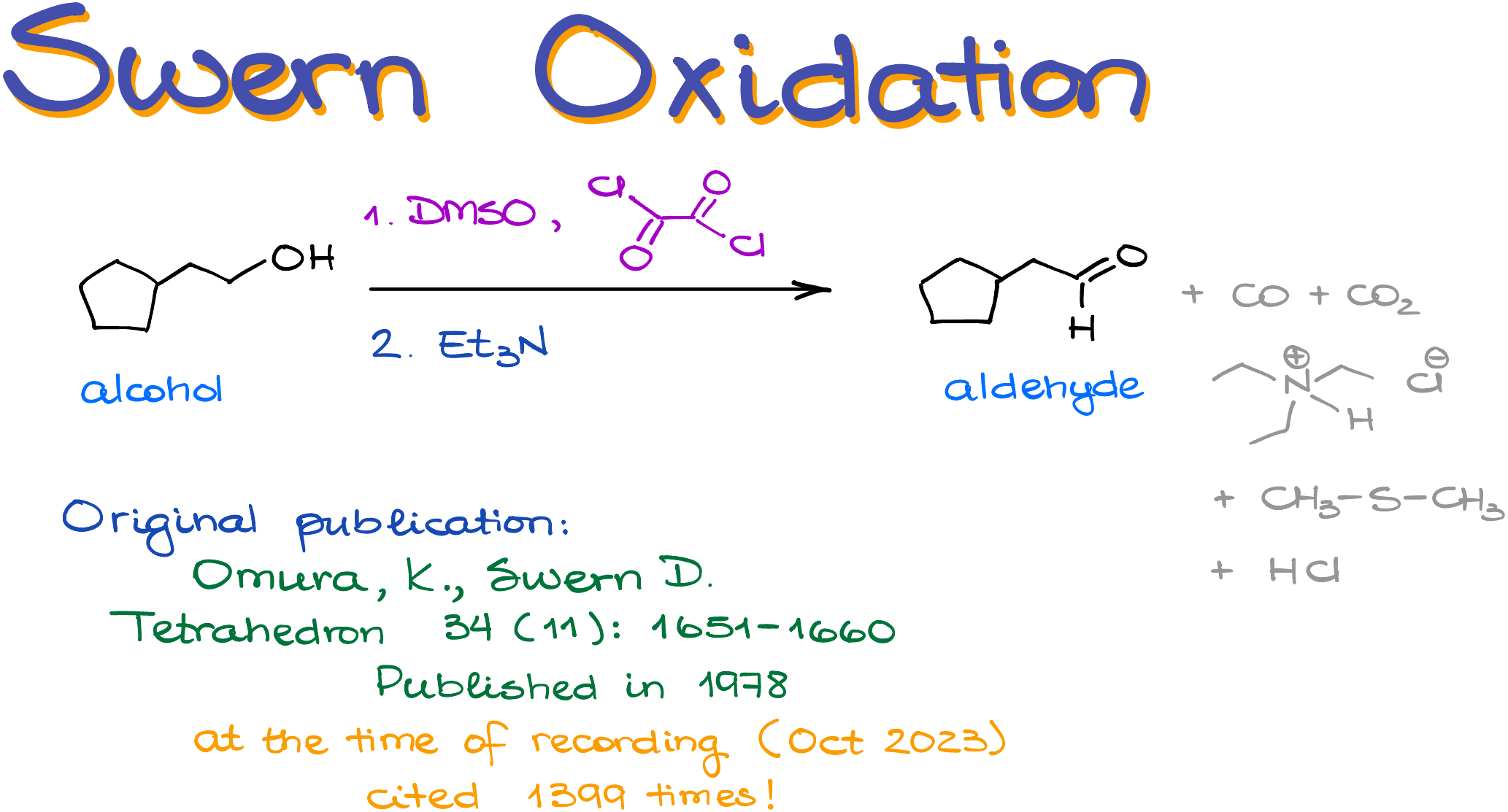
During the 1970s, a decade marked by advancements in oxidation reactions, Daniel Swern developed what became a staple in organic chemistry courses. During the same period, many DMSO-based methods also emerged. Albright–Goldman oxidation that uses DMSO and acetic anhydride, Parikh–Doering oxidation oxidation that uses DMSO and sulfur trioxide (SO3), and Pfitzner–Moffatt oxidation that uses DMSO and carbodiimides like DCC to name a few. Yet, the Swern oxidation is the one that stole the heart of the textbook authors, so we mainly teach that one out of the entire family of similar reactions.
In this tutorial we’ll go over the intricacies of the Swern oxidation mechanism and the important points you need to keep in mind for the exam. So, let’s jump into the mechanism right the way!
Mechanism of the Swern Oxidation
The Swern oxidation proceeds in two unrelated phases. First, we need to create the sulfonium cation that will react with our alcohol. We make this sulfonium ion by reacting DMSO with oxalyl chloride.
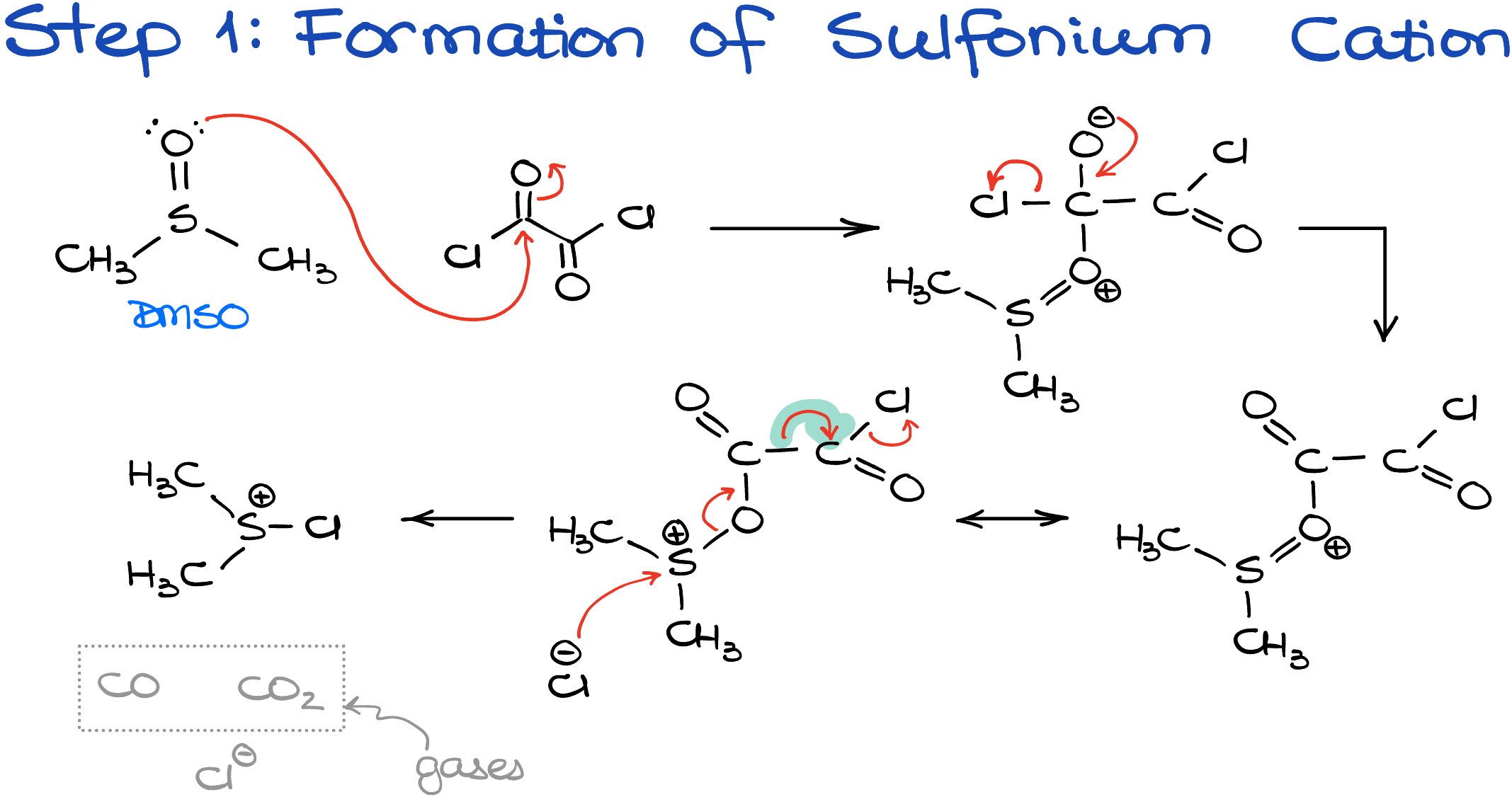
The first step of this reaction is a typical nucleophilic attack from the DMSO onto the acyl chloride functional group resulting in the replacement of the chlorine atom with the sulfur-containing group. This intermediate quickly decomposes forming the dimethylchlorosulfonium chloride and a ton of gaseous products like carbon monoxide and carbon dioxide.

While this is not the only method of forming the sulfonium ion, this is the most common one. You may also encounter a reaction of dimethylsulfide with chlorine NCS (N-chlorosuccinimide) as some alternative ways of getting there. And frankly, I don’t blame chemists for looking for alternative ways, coz’ the oxalyl chloride is one of those lovely reagents that you’ll hate from the first whiff! Although, the dimethylsulfide is also known as one of the worst smelling chemicals out there, so I don’t honestly know which one is worse.
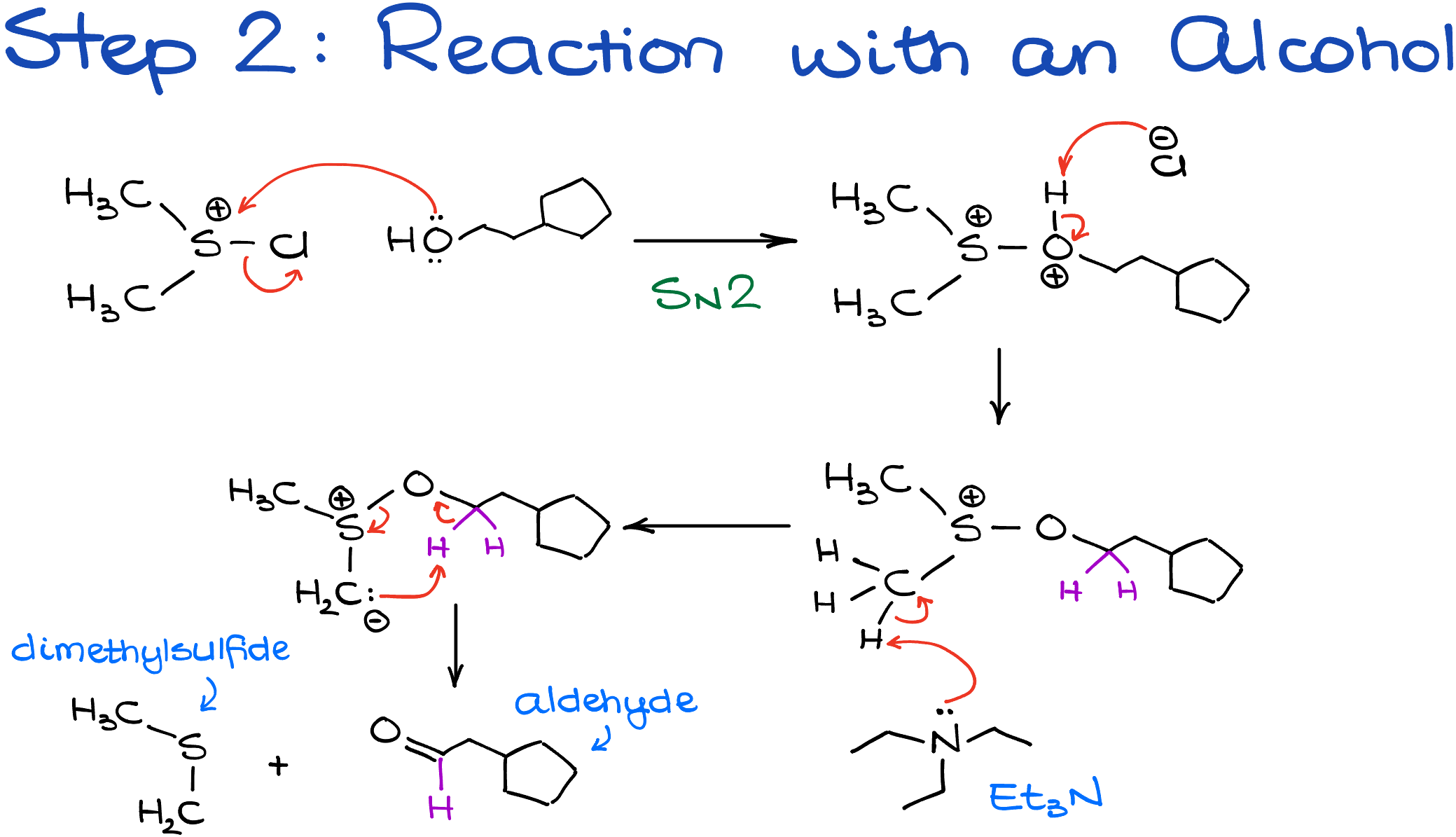
The sulfonium ion that we have prepared in the first step of this reaction rapidly reacts with the alcohol. This reaction makes an intermediate with an oxygen-sulfur bond which we are going to treat with a base. Typically, the base of choice here is triethylamine, although other alternatives are also possible.
Now, there’s a correct way to show the next step in this mechanism and there’s an incorrect one. Some textbooks and instructors want to show this step in an analogous way to other oxidations that you’re most likely have seen up to this point, namely Jones and PCC. So, they go after the ⍺-hydrogens right the way. However, the research data doesn’t support that pathway. It has been shown in a number of studies that triethylamine first deprotonates the methyl group attached to the sulfur atom. Only then this newly formed anionic species undergoes the intramolecular proton transfer giving us the final product and dimethylsulfide as a co-product.

While this mechanism is kinda long and the first part of it you have to pretty much memorize, it is a commonly tested mechanism. And while I cannot guarantee that your instructor will bring it on the test, if you cover the Swern oxidation, there’s a high chance it will show up on the test as well. So, better be safe than sorry when it comes to fancy reactions like this one.
Examples of the Swern Oxidation
The swern oxidation, like many other oxidation reaction of this type, have excellent chemoselectivity. For instance, in this example, we are going to oxidize the primary alcohol and nothing happens to our carbonyl.
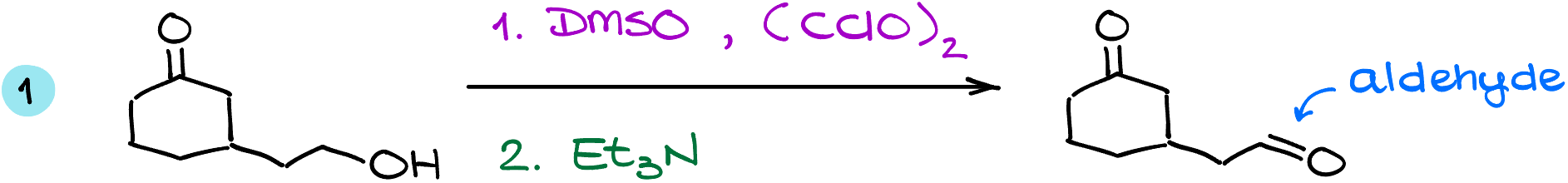
In this example, I have a molecule with two primary alcohols and an alkene. Here, the oxidation gives me a dialdehyde and keeps my double bond intact.
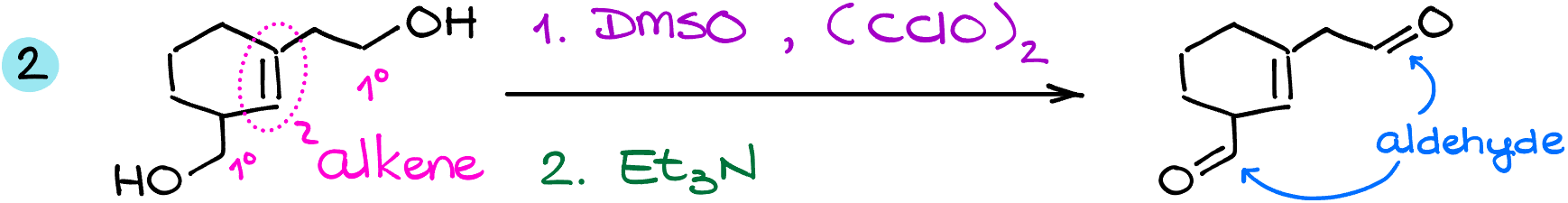
And finally, in this case I have a molecule with an secondary alcohol and a primary alcohol. Here, the primary alcohol will turn into the corresponding aldehyde. While the secondary alcohol will give us a ketone.