Meso Compounds
In this tutorial I wanna talk about probably one of the most confusing terms in stereochemistry — meso compounds. Many students have a lot of misconceptions about the meso compounds, but I can guarantee you that by the end of this video you’ll know everything there is to know about the meso compounds, how to identify them, and what to look for on the test.
Definition of a Meso Compound
The official definition of a meso compound is the following:
“A meso compound is an achiral isomer in a set of stereoisomers, at least two of which are chiral.”
It’s quite mouthful. What does it even mean? Does it mean that we need to know all possible stereoisomers to identify which one is meso? Absolutely not! And since this definition is kinda hard to apply, we typically see a different definition:
“A meso compound is an achiral molecule that has at least two chiral atoms.”
Well, that is much easier to stomach! So, a meso compound is a substance that fits two criteria:
- It needs to have two or more chiral atoms.
- It needs to be achiral, aka superimposable with its own mirror image.
This means that the meso compound is a sub-class of achiral molecules.
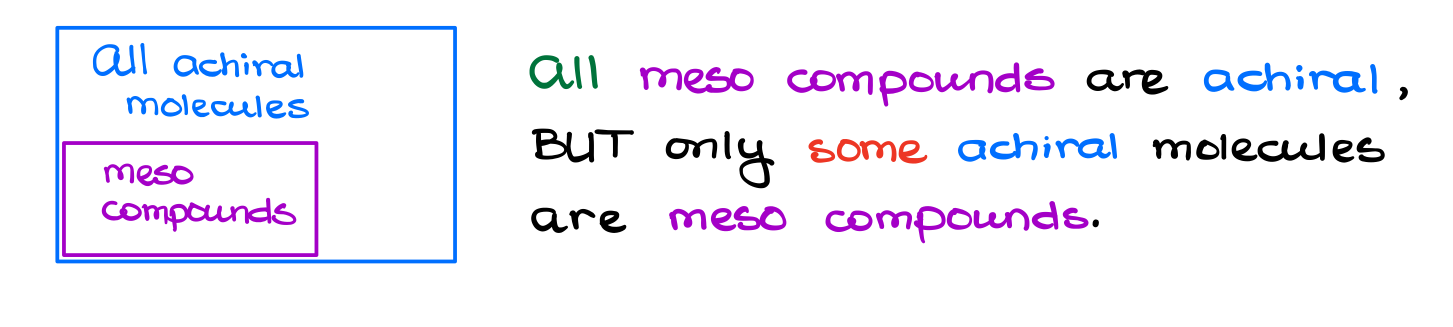
The first criterion is easy. A chiral atom needs to have 4 different groups on it. And we need to have at least two of those guys. Notice how I purposefully say “atom” instead of “carbon?” I’m doing it quite intentionally, as other atoms can be chiral as well. Even in an introductory organic chemistry course we can see chiral nitrogen, phosphorus, or sulfur. Of course, carbons are going to be our common targets here.
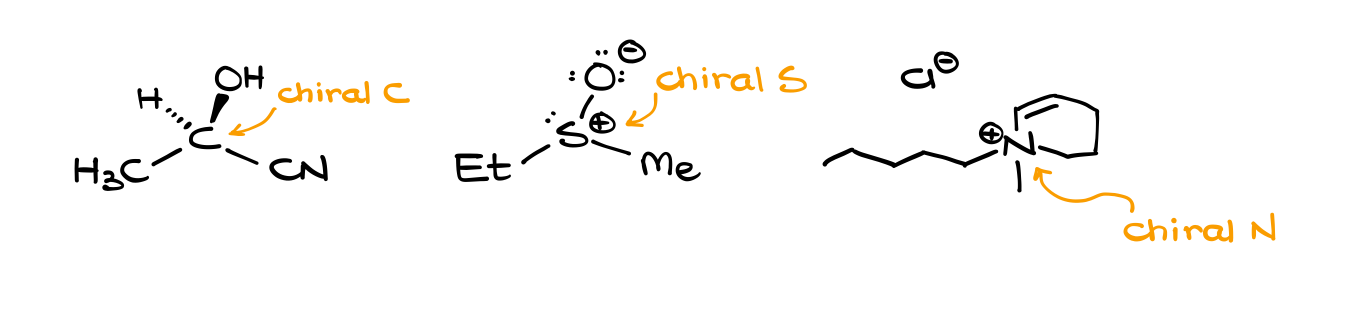
I also want to remind you that we do not limit ourselves to just the first atom when we are analyzing our groups attached to our atom of interest. You need to take the entire group into consideration. Groups might be different later in the chain. And if you don’t pay attention to those differences or simply discard those, you can easily misinterpret your molecule and miss the chiral atom.

Now, the second criterion is the mirror image. This one might be a bit more challenging as it actually requires you to draw the mirror image itself, and then try to rotate it around to superimpose it with the original molecule. Notice how I said “draw it” and not just “imagine it.” Again, I’m doing it on purpose. Do not be complacent in your ability to imagine molecules in 3D. As the matter of fact, my experience shows that the absolute majority of people are quite bad at it. It takes time and deliberate effort and practice to develop the 3D ability. So, don’t be lazy and draw the molecule.
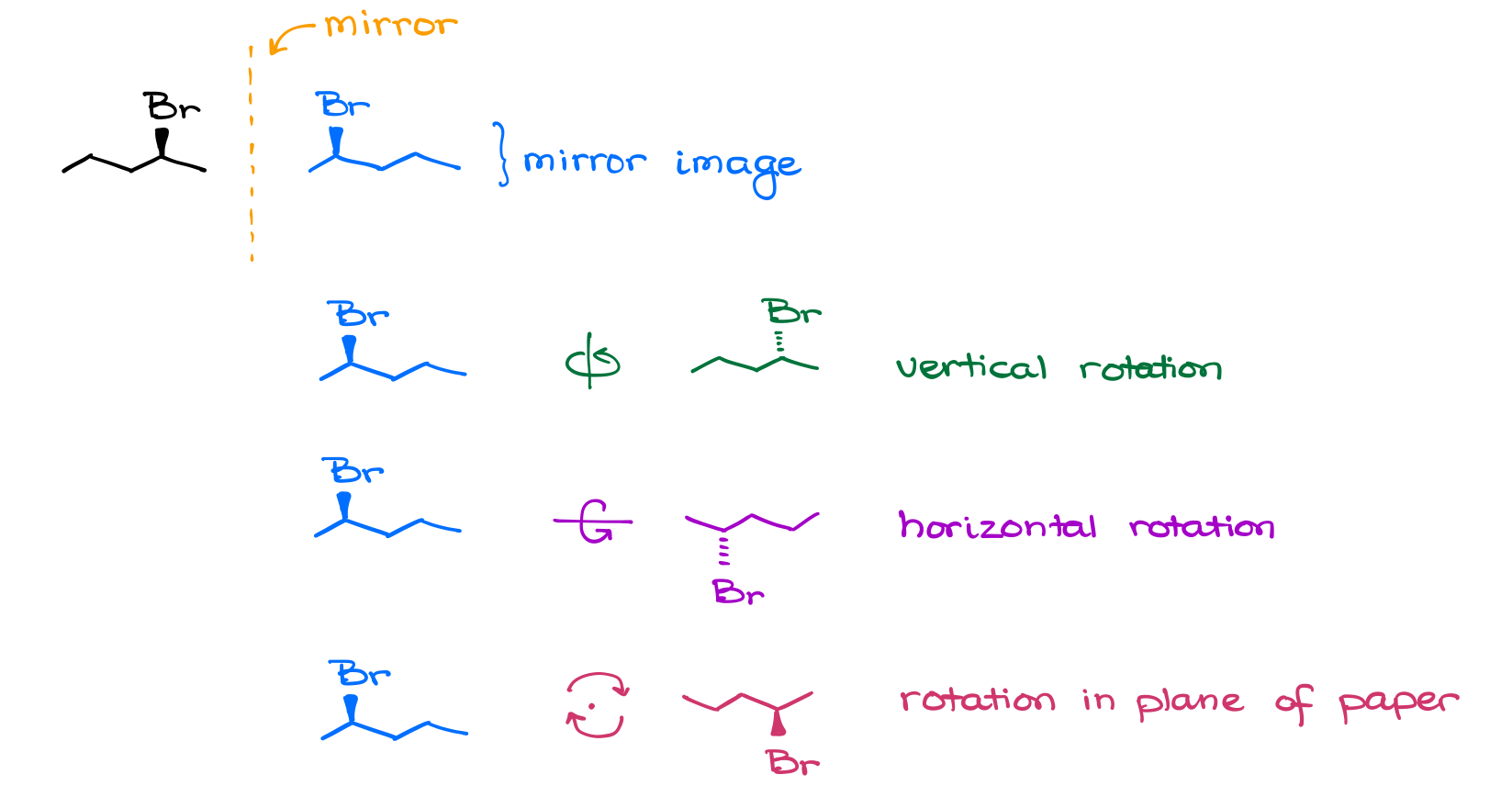
Once you have your mirror image, there are 3 rotations in space that you can do to see if it superimposes with the original molecule or not.
- Rotation in the vertical plane.
- Rotation in the horizontal plane.
- Rotation in the plane of paper.
These three are the must-know operations, and you should practice to make sure you can do them correctly.
Plane of Symmetry
A common feature of many meso compounds is a plane of symmetry. If your molecule has an even number of chiral atoms and it has a plane of symmetry cutting your molecule through the middle, you’re looking at a meso compound.
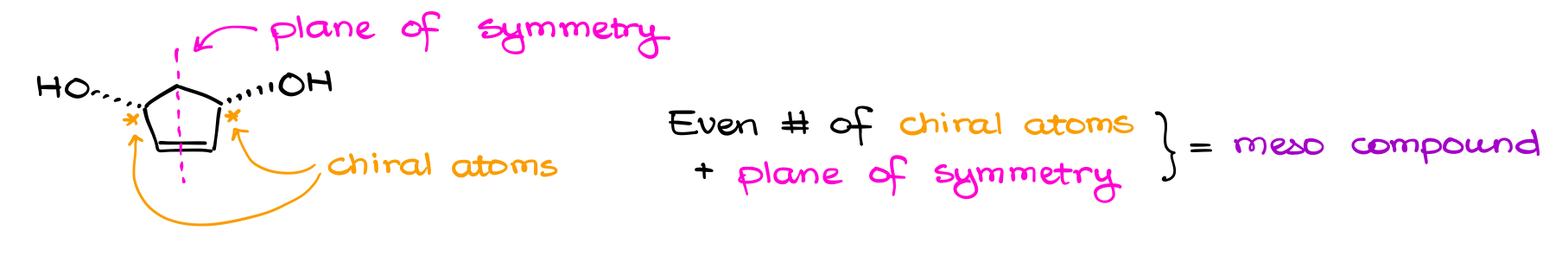
It’s important to keep in mind though, that to have a plane of symmetry, you need to have a very specific arrangement of atoms in your structure. A molecule with a plane of symmetry will look like an inkblot or a butterfly.
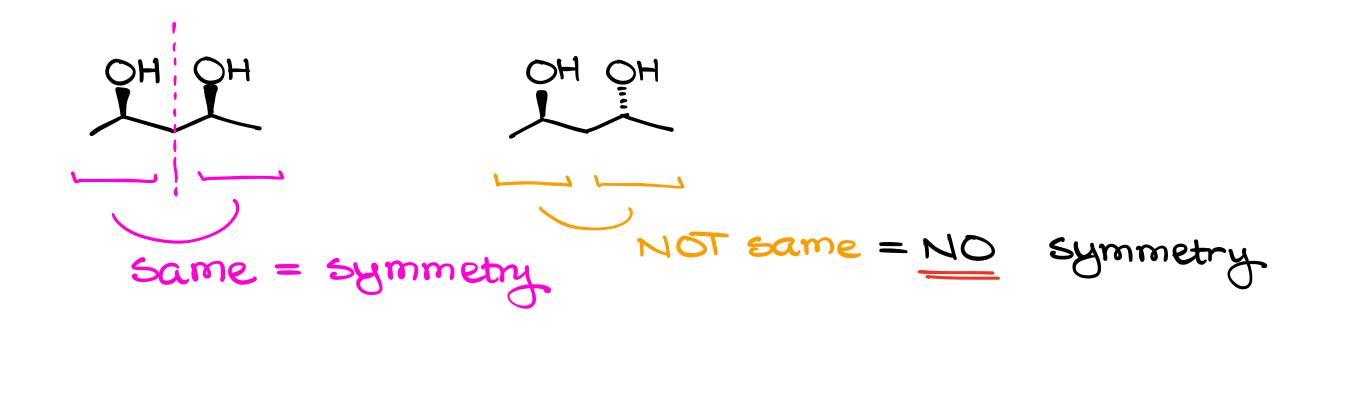
But while many meso compounds do have a plane of symmetry, it’s not a requirement! The definition didn’t mention a plane of symmetry at all. So, it means that we can potentially have molecules that do not have a plane of symmetry, and yet they are meso. There’s a definition of meso compounds that you might encounter that states that the meso compound is superimposable with its own mirror image due to the plane of symmetry. That’s actually only partially correct. Meso compounds superimpose with their mirror image due to the element of symmetry, and not every element of symmetry is a plane. We can have meso compounds with an inversion axis, which is an element of symmetry but it’s not a plane of symmetry.
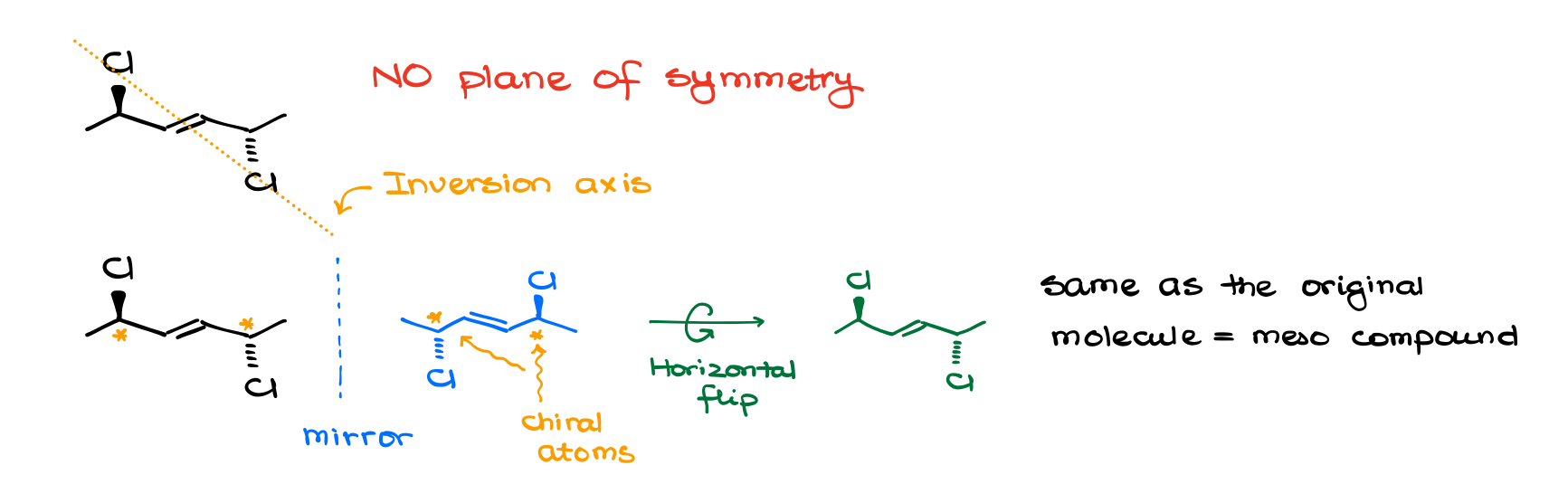
So, don’t blindly rely on a plane of symmetry in your determination. Likewise, just because a molecule does have a plane of symmetry, it doesn’t make it a meso compound. Remember, that a meso compound needs to have chiral atoms. So, a symmetrical molecule without chiral atoms is not going to be meso.
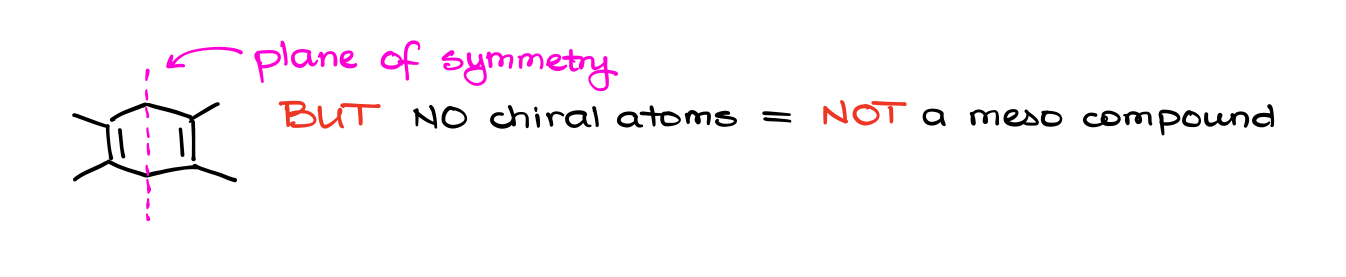
This is a common mistake that I see a lot of students make, so be very careful and don’t fall into this trap on the test.
