Hydroboration-Oxidation of Alkynes
In this tutorial, I wanna talk about the hydroboration of alkynes, which is like a dragonfly of organic reactions: it’s precise, it’s efficient, and it’s one of the coolest reactions of alkynes, just like the dragonfly is one of the coolest insects.
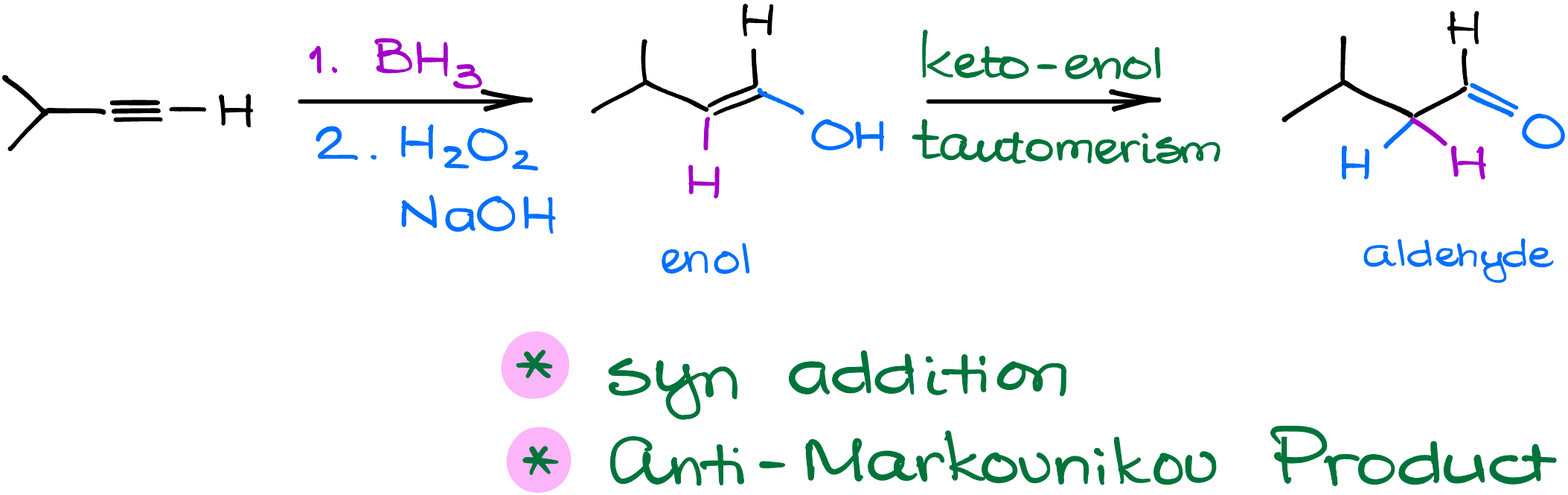
Mechanism of the Hydroboration Reaction
Let’s dive into the hydroboration of alkynes using our example, 3-methylbut-1-yne.
First, borane attacks the alkyne’s pi-bond, much like what we see in the hydroboration of alkenes. A hydrogen simultaneously bonds with a carbon atom. Because this process is a syn addition, the hydrogen and boron end up on the same side of the newly formed double bond.
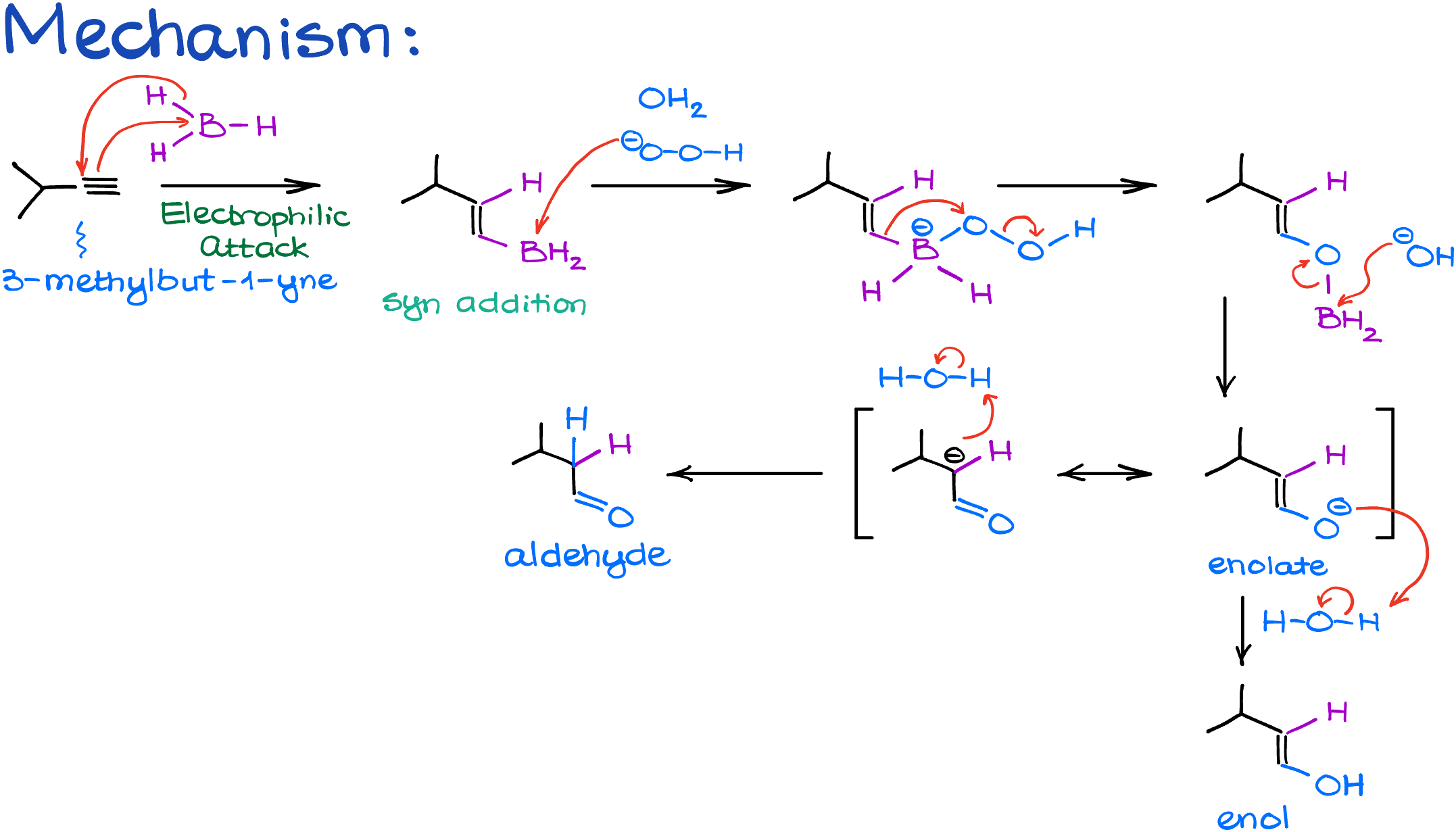
Now, let’s keep things simple. Even though borane can sometimes react with multiple alkynes, we’ll focus on it reacting just once.
Next up is the oxidation stage with hydrogen peroxide. A hydroxide ion removes a proton from the hydrogen peroxide, forming a peroxide anion and water. This peroxide anion then targets and binds with the boron. Following this, the carbon shifts onto the oxygen atom, allowing us to then remove boron and obtain an enolate ion. This ion benefits from resonance stabilization, with the primary resonance placing a negative charge on the oxygen.
When we introduce a proton to this molecule, it can bond either with the carbon or the oxygen. If it chooses the carbon, we get an aldehyde. If it picks the oxygen, we end up with an enol. Think of this step as the keto-enol tautomerism. The aldehyde takes the lead because it’s more stable overall. Since the keto-enol tautomerization is an equilibrium process, we always prioritize the formation of the more stable product—aldehyde in this case.
This way, you can essentially bypass the formation of the enol altogether. Although, it might be a good idea to keep it in mind for the purposes of a simple visualization of the products.
In Short: Through a series of precise steps – borane attack, oxidation, and tautomerism – we easily turn an alkyne into an aldehyde product.
Shortcut to Predict the Product Easily
When predicting the product for hydroboration reactions, consider the example of cyclohexylethyne. Redraw the molecule with a double bond, adjusting the angle to 120° due to the change in carbon hybridization. Add an -OH group to the less substituted carbon, resulting in an enol. Use the hydration of alkynes technique to transform this into the final aldehyde product by adjusting the double bond to a carbonyl.
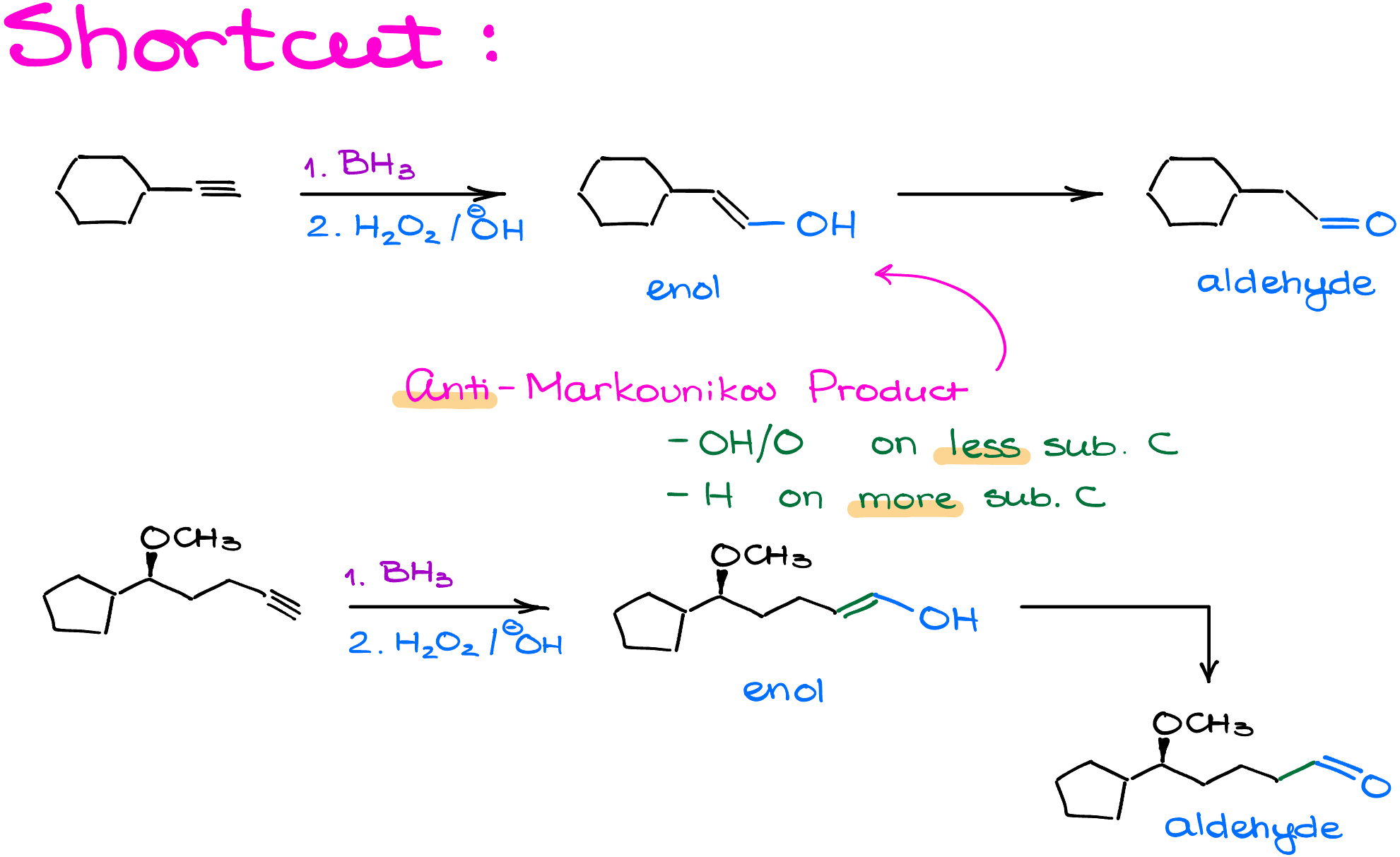
This anti-Markovnikov product places the -OH/O on the less substituted carbon and the hydrogen on the more substituted carbon.
Hydroboration of Alkynes with Internal Triple Bonds
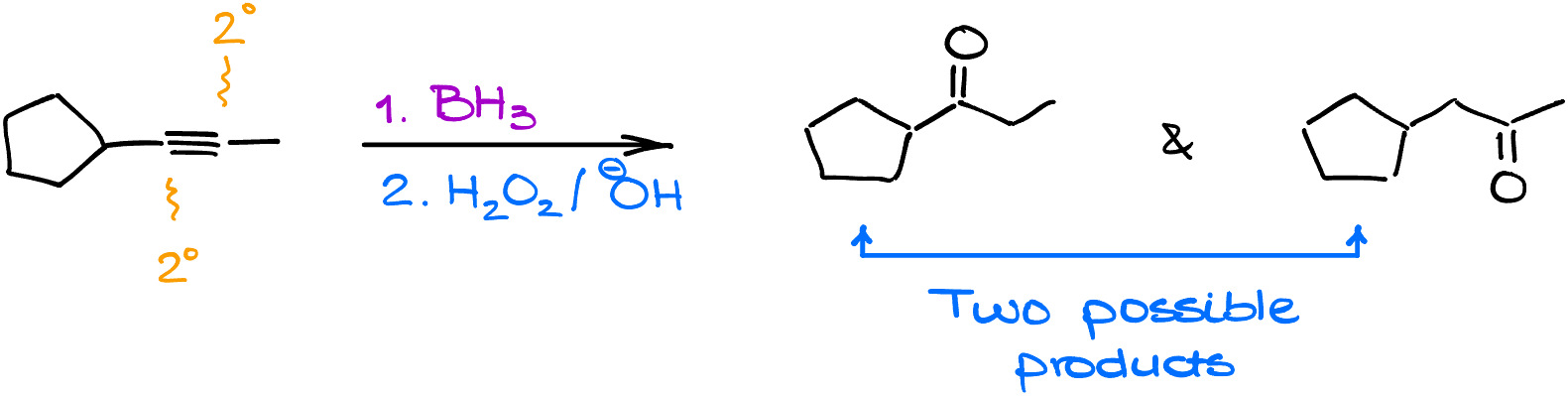
Handling a molecule with an internal alkyne presents challenges since both atoms are 2°, making it hard to distinguish a more or less substituted atom. Regular hydroboration-oxidation would give a mixture of products. However, instead of a standard borane, bulky boranes like disiamylborane, 9-borabicyclo[3.3.1]nonane (9-BBN), or dicyclohexylborane can be employed.
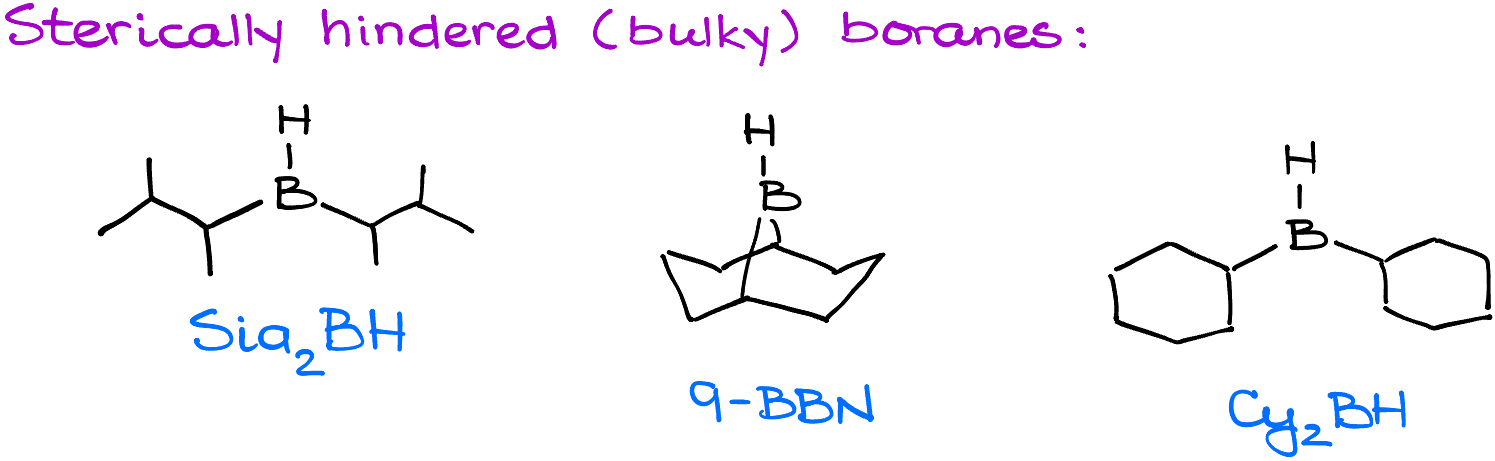
These reagents, being sensitive to steric hindrances, align in a way that places the boron with bulky groups away from the larger group of the substrate. This alignment ensures the final product has the oxygen on the atom further away from the bulky group.
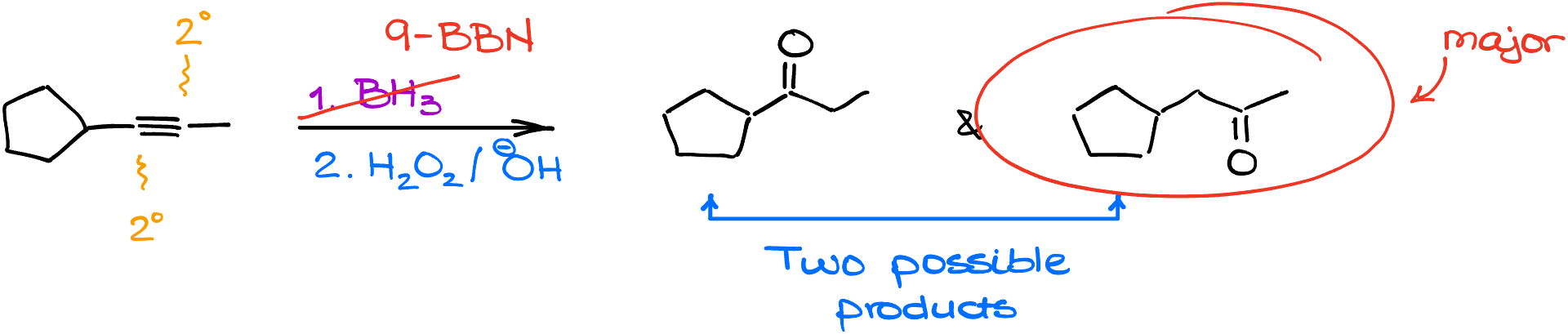
Using 9-BBN instead of regular borane, for instance, results in a major product where the borane orients away from larger groups like the cyclopentyl ring during the reaction’s initial step. This orientation creates an intermediate with groups spaced further apart. Consequently, the product has the -OH group and the resulting oxygen atom positioned further from the cyclopentyl ring. So, with the right reagent, the challenge of internal alkynes can be navigated effectively, yielding the desired products.
So, remember, if you need additional regioselectivity in your hydroboration reaction, use one of the bulky boranes. And you can also use this same trick in the hydroboration of alkenes reaction to make sure your alcohol always ends up on the less substituted atom.
I bet the first time you saw a mechanism like this, it seemed intimidating. Well, how about now, especially when you know an easy trick to predict the final product? Is it still a subject of nightmares or you feel like you can do it? Tell me what you think in the comments below!
