SN1, SN2, E1, E2 Predictive Model: How to Decide Which Mechanism We Have
In this tutorial, I wanna summarize the substitution and elimination reactions and show you the predictive model that you can use to determine the mechanism of your reaction. If you haven’t seen my videos on the SN1, SN2, E1, and E2 mechanisms, I strongly encourage you to first watch those and then come back to this video.
So, let’s start by a quick review of the important features of each mechanism.
The SN1 reactions are unimolecular, and their rate only depends on the concentration of the substrate, typically alkyl halide.
The rate-determining step in the SN1 reaction is the carbocation formation. And the carbocations are the major sources of all our troubles with the SN1 reactions. Because of the carbocation intermediates, we are going to see the racemization of the product and possible carbocation rearrangements. Also, since 1° carbocations and CH3+ are too unstable for our purposes, we are not going to see SN1 reactions with 1° substrates unless they are resonance stabilized.
The SN1 reactions also require the polar protic solvents like acids or alcohols. The solvent is extremely important in the SN1 reaction as without it there will be no reason for the leaving group dissociation. Additionally, the solvent typically is the nucleophile in the SN1 reaction as well.
The E1 reactions have a lot of similarities with the SN1 reactions as they share the mechanistic pathway and the carbocation intermediate. Typically, E1 reactions are favored at higher temperatures while the SN1 reactions are favored at lower temperatures if all other aspects of the reaction are equal. Additionally, the E1 reactions favor the formation of the more substituted double bond, known as Zaitsev product.
The SN2 reactions are bimolecular reactions, and their rate depends on the concentrations of both the substrate and the nucleophile. They are also concerted processes, meaning that the key step int the reaction is a single step, and we see no intermediates in these reactions. The SN2 reactions have strict requirements to the nature of the nucleophilic attack. Namely, they require back-side attack which causes the inversion of the stereochemical configuration of the atom where we perform the attack. Also, because of the backside attack requirement, the SN2 reactions are extremely sensitive to the steric hindrances and favor substrates with the 1° leaving groups. Finally, the SN2 reactions are favored in the presence of the polar aprotic solvents which help making our nucleophiles more aggressive by solvating the cations.
E2 reactions are similar to the SN2 in the sense that they are also bimolecular processes in which the reaction rate depends on the concentration of the substrate and the base. So, that means that we also require a base in this reaction instead of a nucleophile. Preferably, we want a strong base. And talking of which, we are going to separate our bases into two distinct categories: small bases and bulky bases. This is important because the small bases tend to give the Zaitsev product (the more substituted alkene), while the bulky bases tend to give the Hofmann product (less substituted alkene). Additionally, E2 reactions are sensitive to the conformation of the transition state and require the leaving group and the β-proton to be anti-periplanar to each other. If we can’t find an “anti” proton in the β-position, the E2 reaction is impossible.
Ok, now when we have refreshed the substitution and elimination reactions in our heads, let’s dive into the predictive model itself.
Three Steps in the Predictive Model
The predictive model I propose here is based on the 3-step approaches.
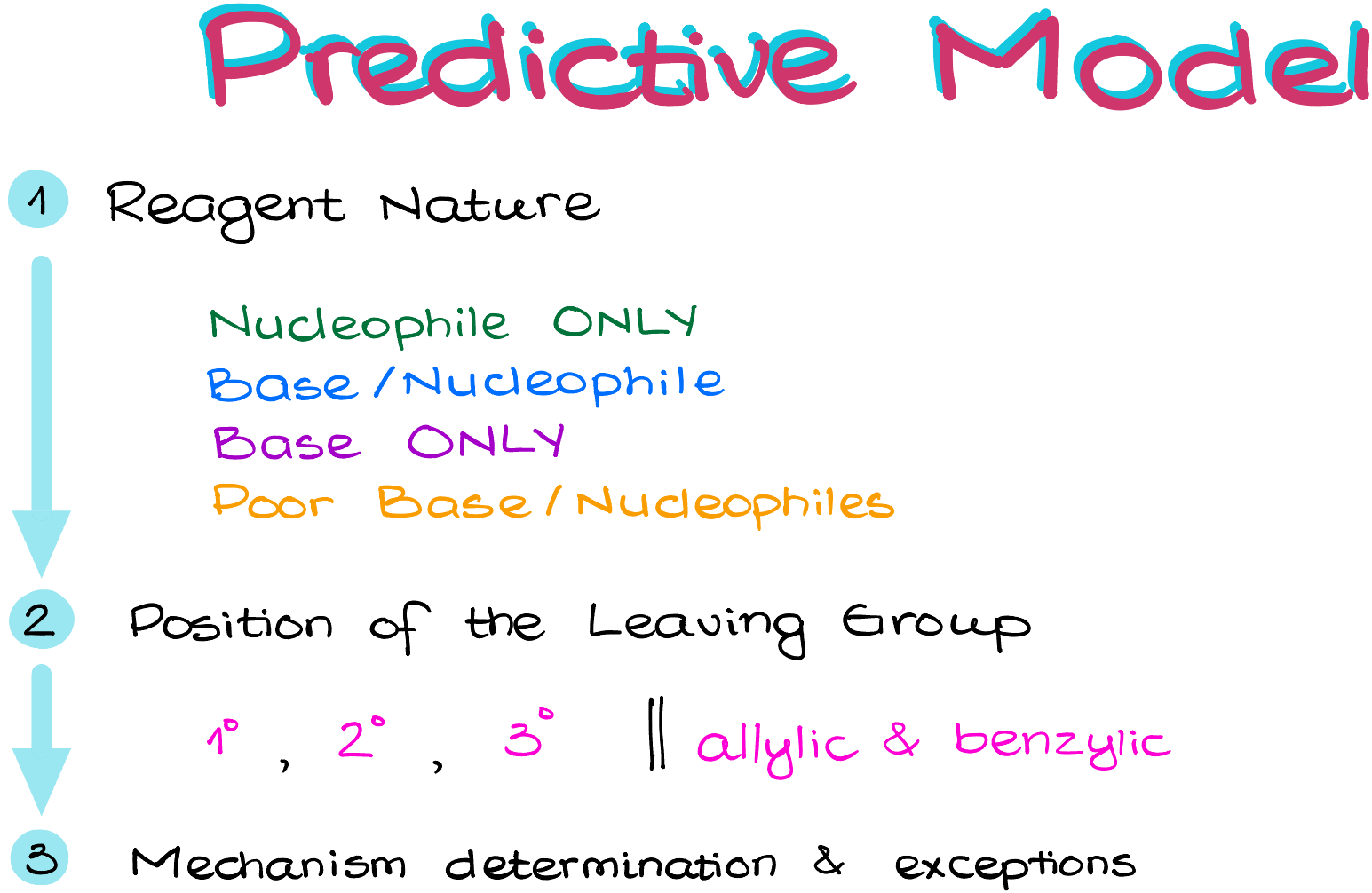
First, we are going to assess the nature of our reagents.
Our reagents will fit into 4 categories: nucleophiles only, nucleophiles/bases, bases only, and poor base/nucleophiles.
Once we categorize our reagent we’ll move to the position of our leaving group.
The leaving groups, of course, can be 1°, 2°, or 3°.
We’ll also specifically point out the allylic and benzylic positions as they have their own intricacies that we must keep in mind.
Once we know all that, we can determine the mechanism by giving this video a thumbs-up and then plugging the pieces into the model and see if we fit any of the exceptions (not that many).
Reagent Nature
Now, let’s talk about those categories in a few more details staring with the nature of our reagents.
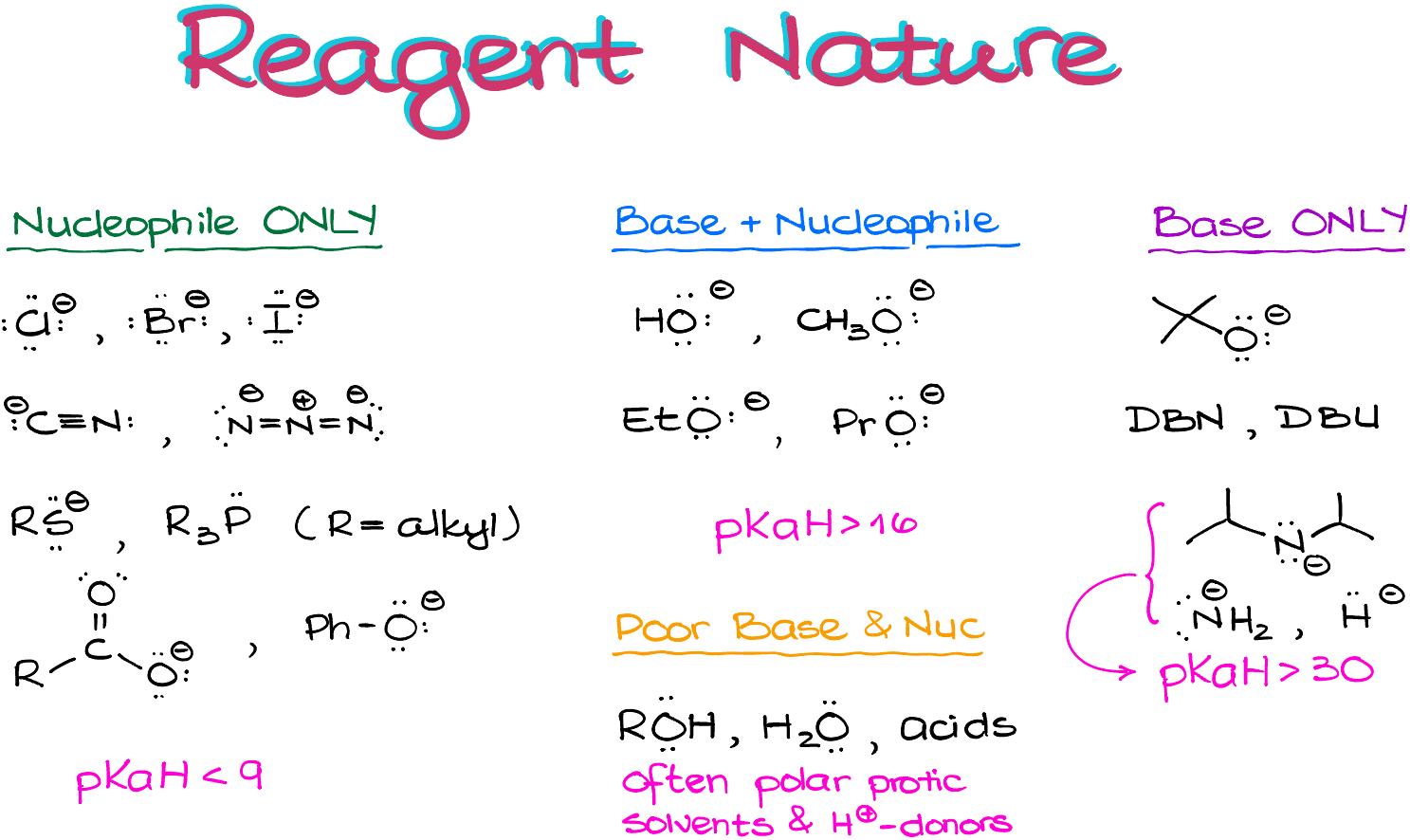
First, let’s look at the “Nucleophile Only” category. Those are typically the ions like halides, cyanide, azide, phosphorus and sulfur-containing compounds, carboxylates, etc. Typically, nucleophile only compounds are the species that have a relatively low pKaH values (pKa of the conjugate acids). I suggest you keep the rough pKaH cut-off at around 9 or so. So, anything with the conjugate acid stronger than that is not very basic and is unlikely to full off a proton from a non-acidic compound.
Nucleophile and Base category typically includes non-sterically hindered alkoxides like methoxide, ethoxide, etc. They also have pKaH values over 16 but under 30.
“Base Only” species are typically either the ions that are extremely basic and will pull off a proton much faster than doing a nucleophilic attack (pKaH over 30), such as amides, or species that are too bulky to be good nucleophiles, such as tert-butoxide.
Finally, the last category is the “Poor Bases and Nucleophiles” which includes acids, alcohols, and other similar species.
It’s a good idea to memorize these categories, so I suggest you copy them down for the future reference.
Using the Predictive Model
When it comes to the leaving groups, they can be 1°, 2°, and 3°.
So, if we add the reagent type to this predictive model, we end up with the 3×4 table of possible outcomes looking like this:
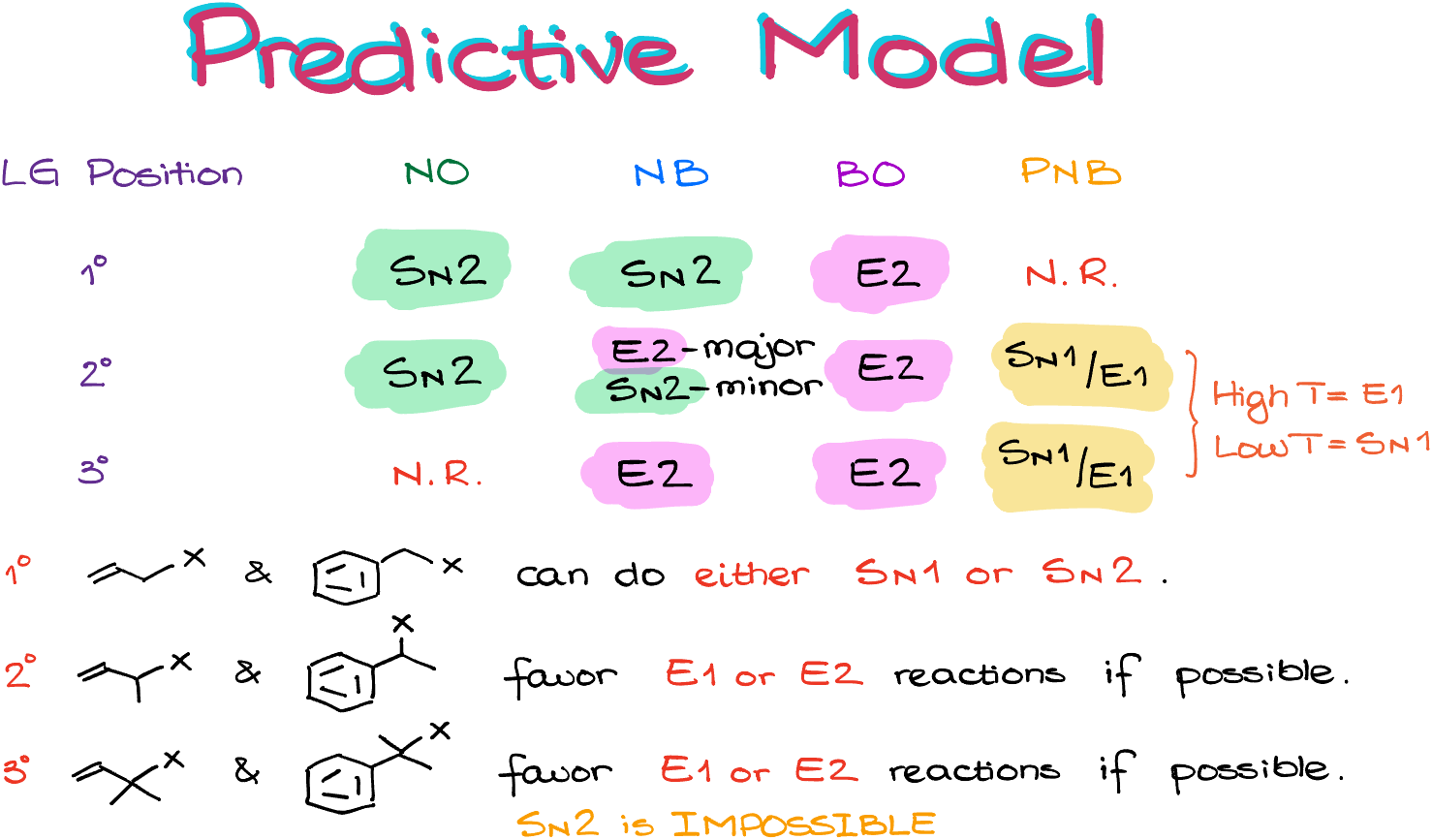
As you can see, it makes a nice diagonal design which is easy to remember.
When it comes to the case where we have competing SN1 and E1 reactions, we are going to look at the temperature of our reaction. Elevated temperatures promote E1 reactions, while lower temperatures favor SN1 reactions. Remember to ONLY look at the temperature if you’re deciding between the SN1 and E1 reactions. It’s only going to matter in those cases. In all other cases, temperature will have very marginal effect.
Now, remember, I have mentioned we’ll talk about the allylic and benzylic positions? They don’t cleanly fit into the scheme, so they their own thing going on.
The primary allylic and benzylic substrates can undergo either SN1 or SN2 reactions. So, it’ll be important to look at the solvent in this case. If we have the polar protic solvent, we’re likely going to be looking at the SN1 reaction. If we have polar aprotic solvent, we’re more likely have a case of the SN2 reaction. The thing is, allylic and benzylic positions can make resonance-stabilized carbocations, which is good for SN1. They are also very reactive in the SN2 reactions due to the molecular orbital interactions in the molecule, plus the lack of the steric hindrances makes SN2 reactions favorable. So, in reality we’re likely have a competition between the two mechanisms.
The secondary allylic and benzylic substrates typically favor the elimination reactions if possible. Due to the additional stability of the resulting conjugated system, the E2 is more favorable than SN2 even with weaker bases. For the same reason, the E1 pathway is more favorable than SN1. However, you would still have to analyze the system and see what the most logical outcome is based on the conditions and the substrate structure. For instance, if there’s no suitable proton for the E2 reaction, the SN2 will be likely in that case. So, in a nutshell, EVERYTHING is a fair game for those substrates.
The tertiary allylic and benzylic positions are similar to the secondary ones. However, the SN2 reactions are going to be impossible due to the steric restrictions.
So, this is the predictive model which will give you the correct answer in the 99% of cases. The only time when it fails is when we have some sort of trickery going on in with the molecule. So, take a screenshot of this scheme, or better yet, copy it down and memorize it. I can guarantee, it will be your saving boat on the test.
You may also have noticed that I barely touched upon the solvents. This is because the solvent is a FACILITATOR of the reaction, and it rarely determines the mechanism itself. So, don’t hang up on the solvent and only look at it at the very end IF you are still unsure which mechanism to pick, like in the case of the allylic or benzylic substrates. In most other cases, the solvent won’t change what’s going on. Rather, it will only make reaction go slower if we have a mismatching solvent.

Last sentence of paragraph 7. Isn’t sn1 favored in polar protic solvent?
Yep, typo! Thank you for noticing, I’ll fix it! That, of course, should’ve been SN2.