Substitution and Elimination Reactions
In this tutorial I wanna look at two types of reactions in organic chemistry: substitution and elimination.
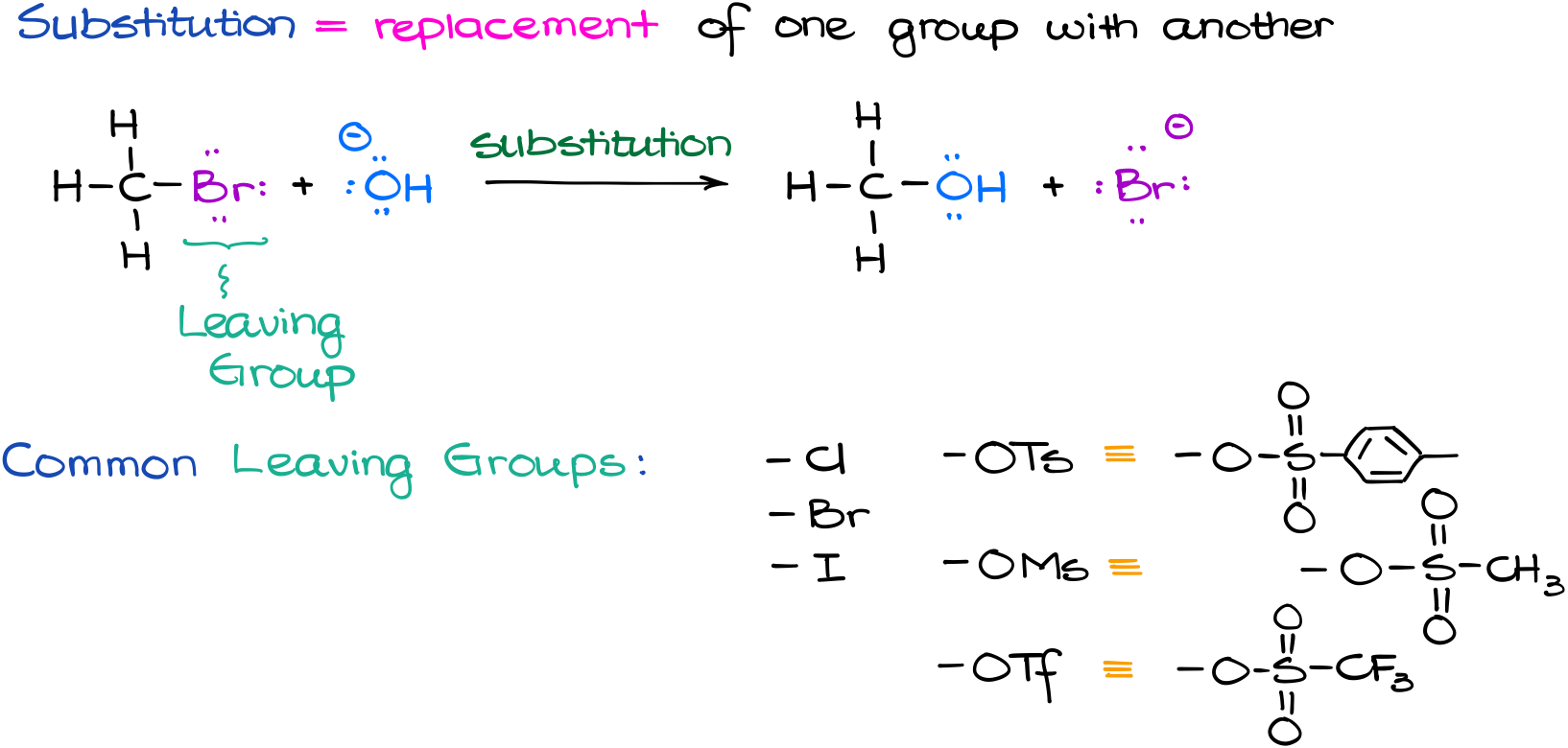
In a substitution reaction we are going to replace a part of our molecule with a different atom or a group. The part of the molecule that is being replaced is called a leaving group because it, well, leaves the molecule. There are many different living groups in organic chemistry, but we will typically see a few most common ones. Those are halogens like chlorine and bromine, or complex one like tosylate or mesylate. The fluorine is not a very good leaving group in most cases, so we are not going to include it in the list of common living groups. And while you might and probably will see some other leaving groups in the future, these are going to be the most common ones you’re going to encounter throughout your course.
An elimination reaction, in contrast to a substitution reaction, is one where we have a loss of a Living Group leading to a formation of a π-bond. And here is something important you want to keep in mind—elimination requires a loss of a leaving group (like bromine or iodine) and one other atom or a group from the adjacent carbon, so we can form a double bond. If you don’t have a suitable group or an atom along with your leaving group that you can eliminate, then your elimination reaction is going to be impossible.
For instance, let’s look at this example here.
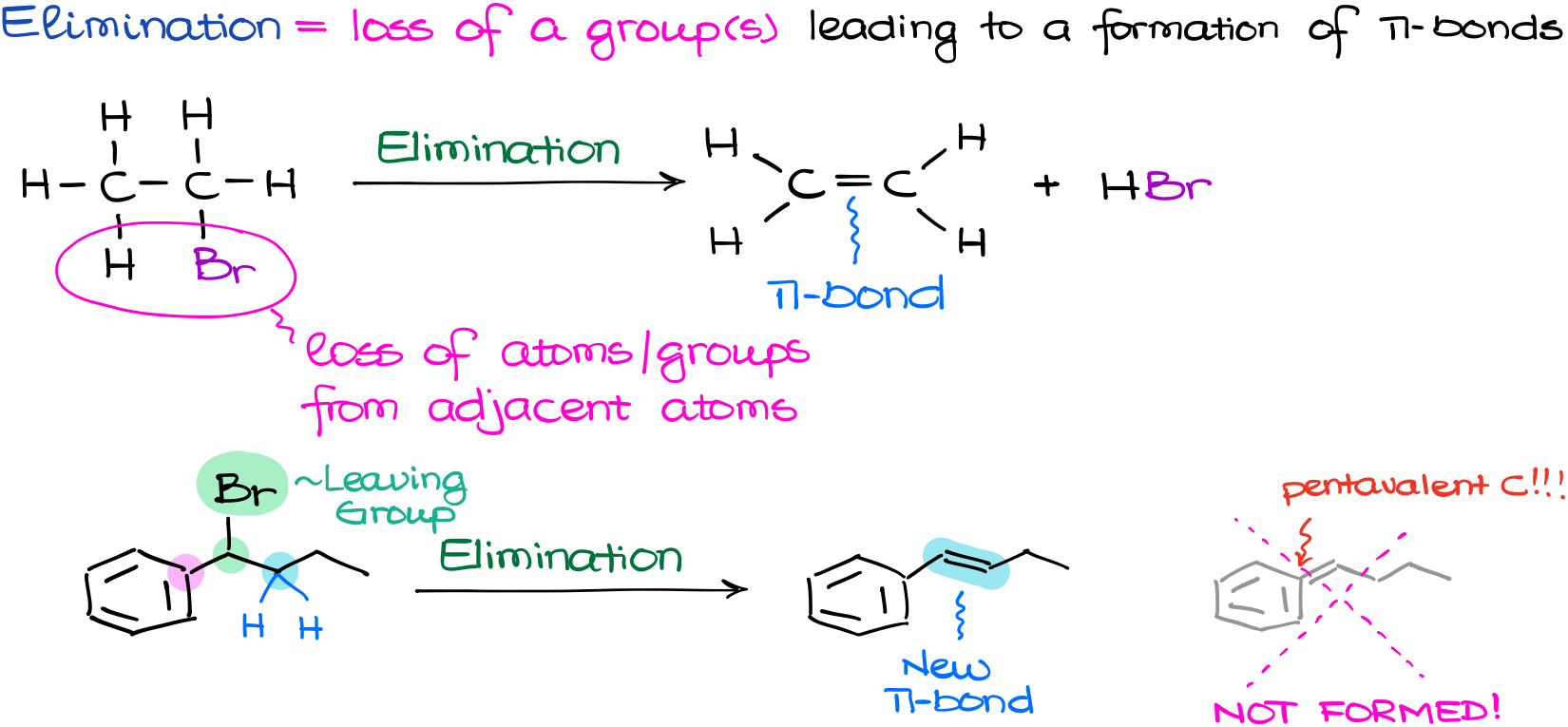
We have our bromine as a leaving group here. However, there are two adjacent atoms to the carbon with a leaving group, let’s mark them as pink and blue. And while we do have a couple of hydrogens on the blue atom, we don’t have anything on the pink atom as it already has four bonds. And remember, carbon can only make four bonds, so there are no implicit hydrogens on that carbon. This means that we only have one direction in which we can form our double bond, which is towards the blue atom. If we attempted to make a double bond in the direction of the pink atom instead, we would end up with a pentavalent carbon, which is sometimes called by its nickname of the “Texas carbon” and this is a huge no-no for us.
In the topics below, we’ll go through the intricacies of each substitution and elimination mechanism. So, get some paper for practice, and let’s get started!
