Bicyclic Compounds
Bicyclic compounds are a fascinating subject in the realm of organic chemistry. These unique molecular structures have essential roles in various chemical reactions, synthesis pathways, and even in the development of pharmaceuticals. Understanding the intricacies of bicyclic compounds, from their different types to the nomenclature rules that govern them, can significantly deepen your comprehension of organic chemistry as a whole.
Classifying Types of Bicyclic Compounds: Systems with Two and Three Bridges
One of the first distinctions to make when studying bicyclic compounds is between the two primary types you’ll encounter: systems with two bridges and those with three bridges.
Systems with Two Bridges
In a system with two bridges, two atoms are connected to each other, forming what is known as bridgeheads. From these bridgeheads emanate two separate bridges, which may vary in size from just a single atom to a sequence of multiple atoms.
Systems with Three Bridges
In a system with three bridges, the bridgeheads remain, but they are no longer directly bonded to each other. Instead, these bridgeheads serve as the starting and ending points for three distinct bridges, which can again range in size.
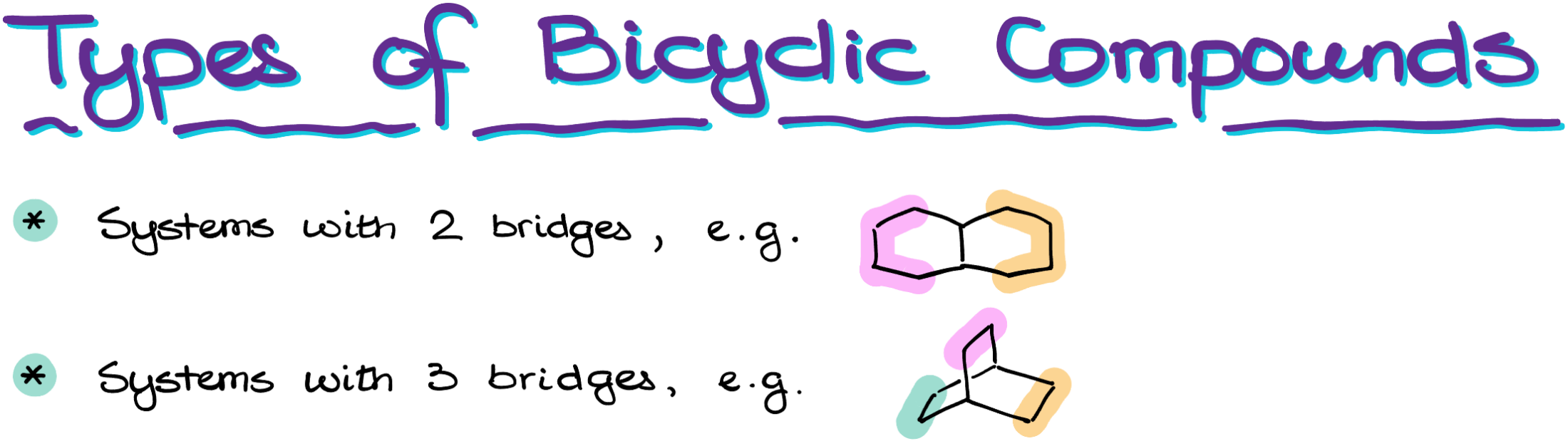
The Art of Naming Bicyclic Compounds: A Step-by-Step Guide
Step 1: Counting the Total Number of Carbons
The initial step in naming a bicyclic compound is to count the total number of carbon atoms in the cyclic structure. This is crucial because the number of carbons will become part of the parent name of the compound.

Step 2: Special Numbering System
Unlike standard nomenclature in organic chemistry, bicyclic compounds have a unique numbering system. Instead of seeking the lowest possible numbers for substituents, the numbering starts from one bridgehead. From this point, the numbering proceeds through the largest loop, then to the next largest, and finally through the smallest loop.
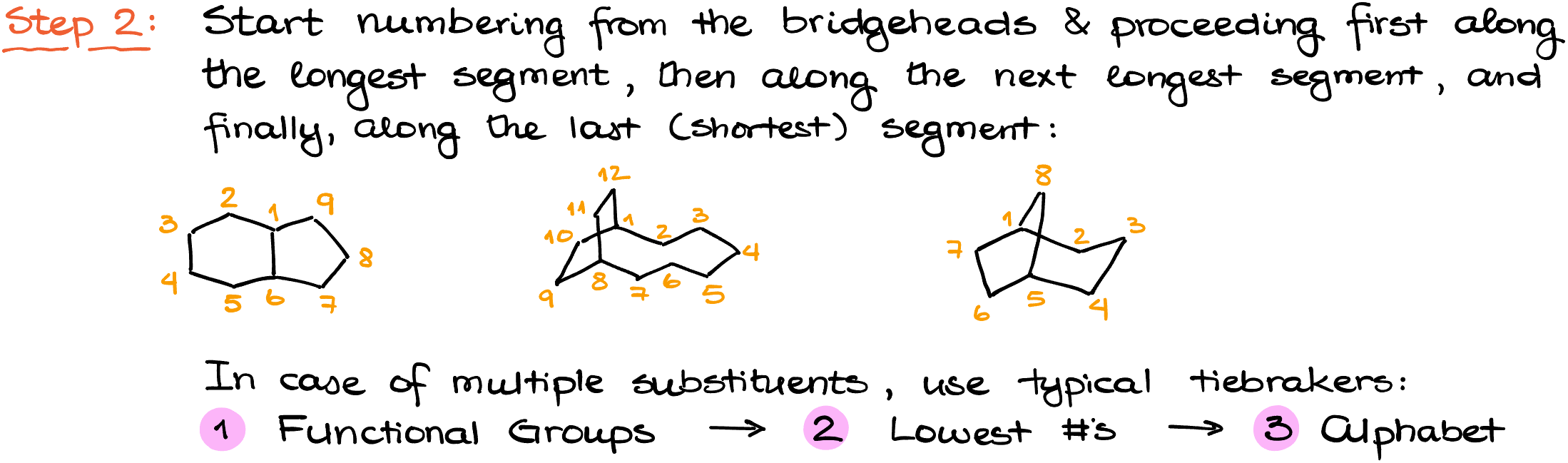
Step 3: Addressing Multiple Substituents & Constructing the Name
If your bicyclic compound has multiple substituents, it’s time to employ some familiar tie-breakers. Functional groups should be given the lowest possible numbers within the numbering scheme. Next, you focus on achieving the lowest numbers for substituents in general. If ambiguity persists, alphabetical ordering serves as the final tie-breaker.
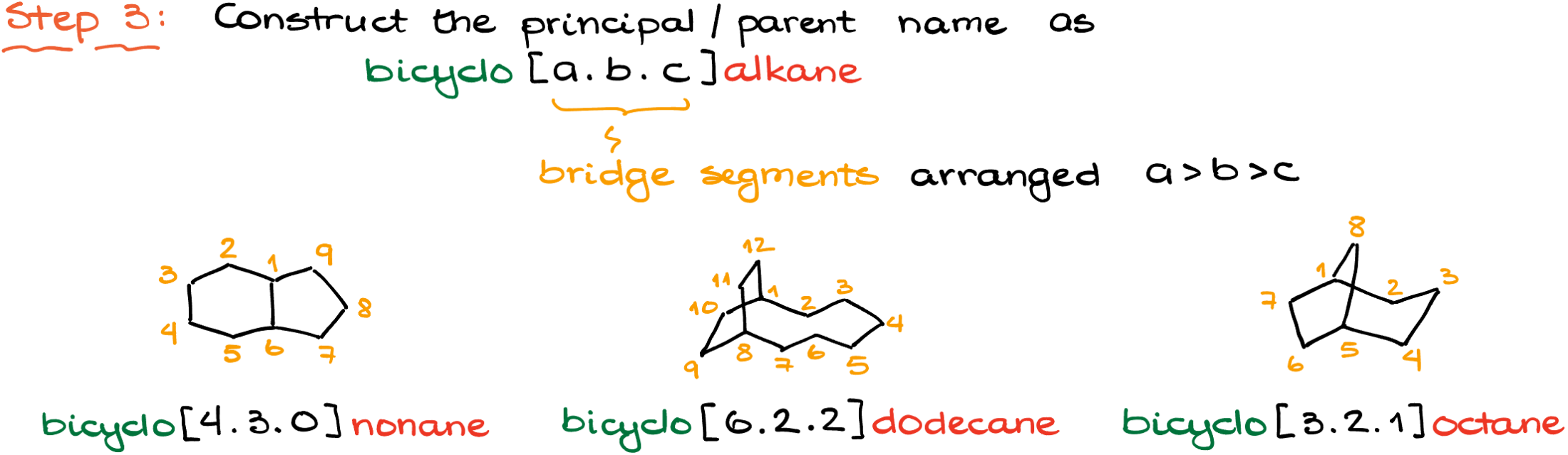
Finally, the name is constructed using a specialized formula. First, alphabetized substituents are listed. Following this is the term “bicyclo,” indicating the bicyclic nature of the molecule. Square brackets then enclose a sequence of numbers separated by dots. These numbers represent the sizes of the largest loop, followed by the next largest, and then the smallest. An essential aspect to remember here is that bridgeheads are not included in counting the size of loops.
By understanding these crucial aspects of bicyclic compounds, from their structural diversity to the rules of their nomenclature, you’ll find yourself better equipped to navigate the complex world of organic chemistry. Now, let’s do some practice to solidify the nomenclature of bicyclic compounds!
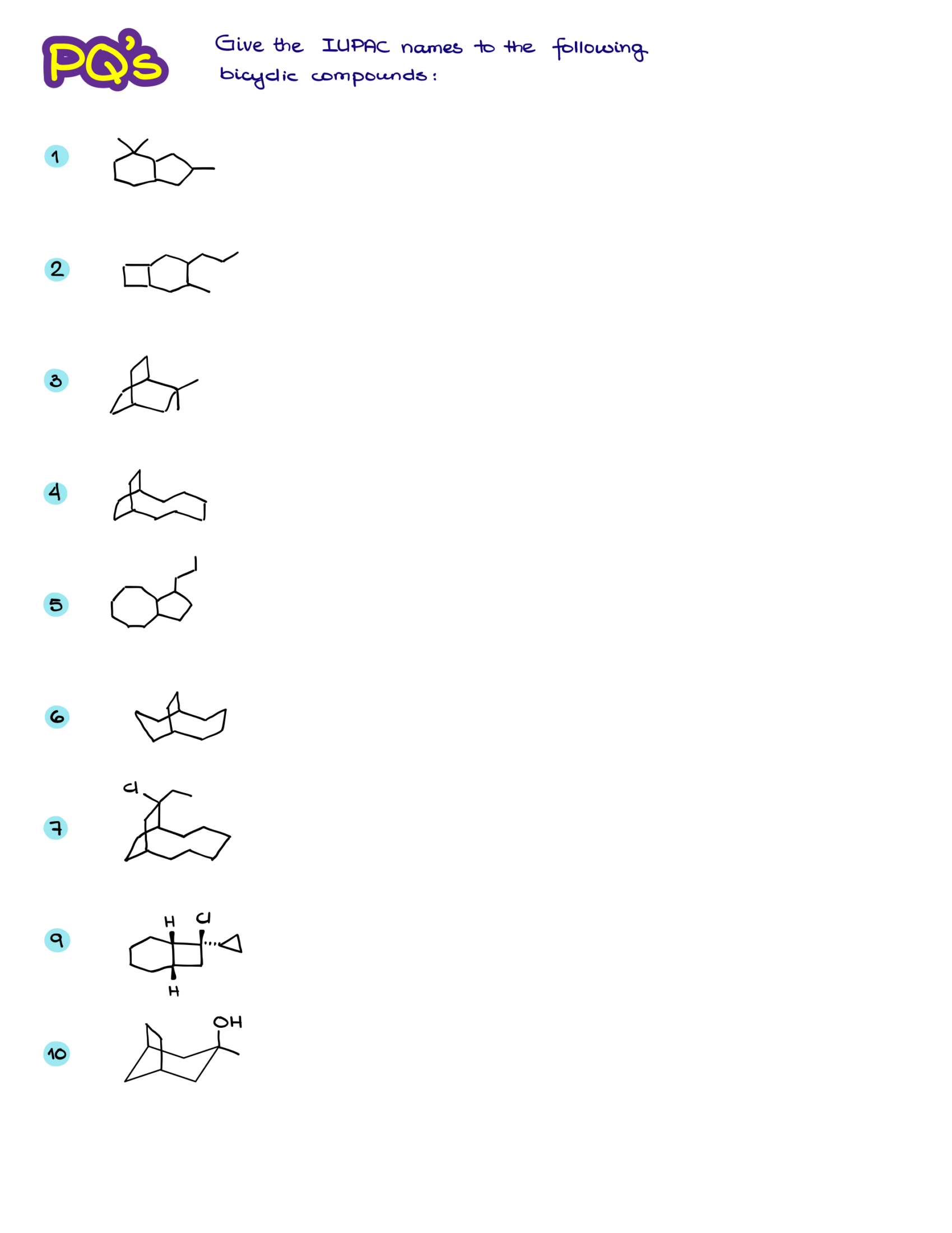
Practice Questions