Resonance
By resonance in organic chemistry we mean an interaction of multiple p-orbitals making a long π-bond spanning multiple atoms. A case of a simple π-bond results from the interaction of the two p-orbitals connecting two atoms. However, there are situations when three or more orbitals can interact making a much longer and much more complex orbital interaction system.
There are 4 types of resonance that you’ll have to know within the scope of your organic chemistry course.
Type 1. An interaction of an empty p-orbital and an adjacent electron pair. That generally results in a structure analogous to a simple π-bond.
Type 2. An interaction between the empty orbital and a π-bond. Those would be examples of allylic and benzylic systems.
Type 3. An electron pair and an adjacent π-bond produce allylic or benzylic anions. We’re not going to focus as much on the anions in organic chemistry as on cations. However, those are very important for certain types of reactivity, particularly, acid-base chemistry.
Type 4. Two (or more) π-bonds interacting with each other. We’ll be talking about those interactions in a lot of details when we discuss the conjugated system. While all the examples above are, strictly speaking, conjugated, we’ll only be referring to the multiple π-bonds participating in resonance as “conjugated system” due to their special chemical properties.
Resonance is one of the most important topic in organic chemistry bonding. It will explain good 3/4 of reactivity in the course, so it’s an extremely important topic to master. Let’s go over those types of resonance in more details.
Empty p-Orbitals Interacting with Electron Pairs
Empty orbitals on any atom will significantly decrease the stability of the atom. In organic chemistry, we generally see empty orbitals on carbons. We call those species carbocations.
As carbocations are intrinsically unstable, they “seek” for any opportunity to fill the empty orbital with electron density to increase their stability. If they don’t have their own electrons, they would have to share those electrons with a nearby atom. In the case when a nearby atom has spare electron pairs, we can share! The typical atoms you’re going to see sharing electrons with empty orbitals on carbons are atoms like oxygen (O) or nitrogen (N). Of course, other atoms with spare non-bonding electrons can do that as well, but the O and N are way more common in organic molecules than, say sulfur (S).
Here’s an example of such an interaction:
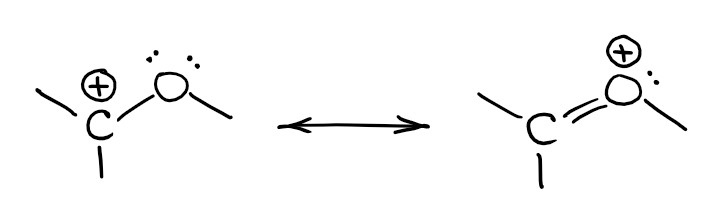
In this case, the electron pair on oxygen will be removed from hybridization and stay on a non-hybridized p-orbital. As the empty orbital on the carbocation is a p-orbital as well, you can have an overlap of the orbitals giving you a new molecular orbital. This new molecular orbital has a lower energy than either of the orbitals making it. In a nutshell, this makes a more stable species. So, from the orbital perspective, here’s what you’re looking at:
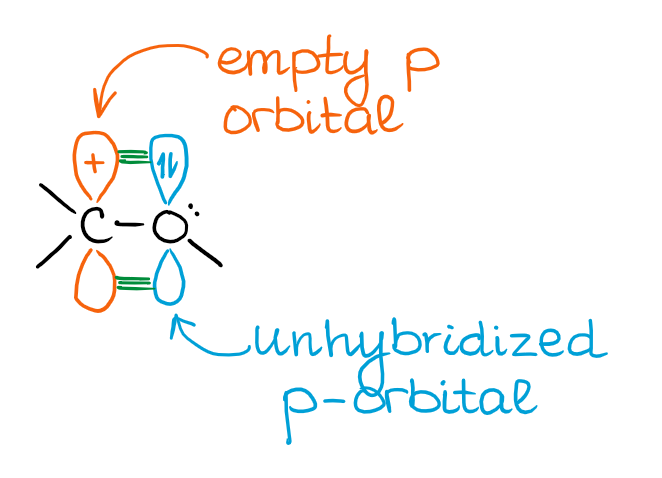
This interaction resembles a π-bond. However, this is a very polar π-bond as each atom doesn’t have enough electrons overall to be neutral.
Note on the Nature of the ⊕ Charge
There’s an important point I want to highlight here: carbocation has an empty orbital, and we use the ⊕ charge to represent that. However, oxygen in the resonance structure to the right does not have an empty orbital! The ⊕ charge on oxygen shows that O is sharing some of its electron density with carbon. But the electrons are still shared and you don’t have the electrons that used to be on O moving completely to C. As the matter of fact, the electron density will still be predominantly residing on the O and the lack of electron density will still be mostly on C.
Examples of Empty p-Orbital Interacting with the Electron Pair
Here are a few example of this type of resonance:
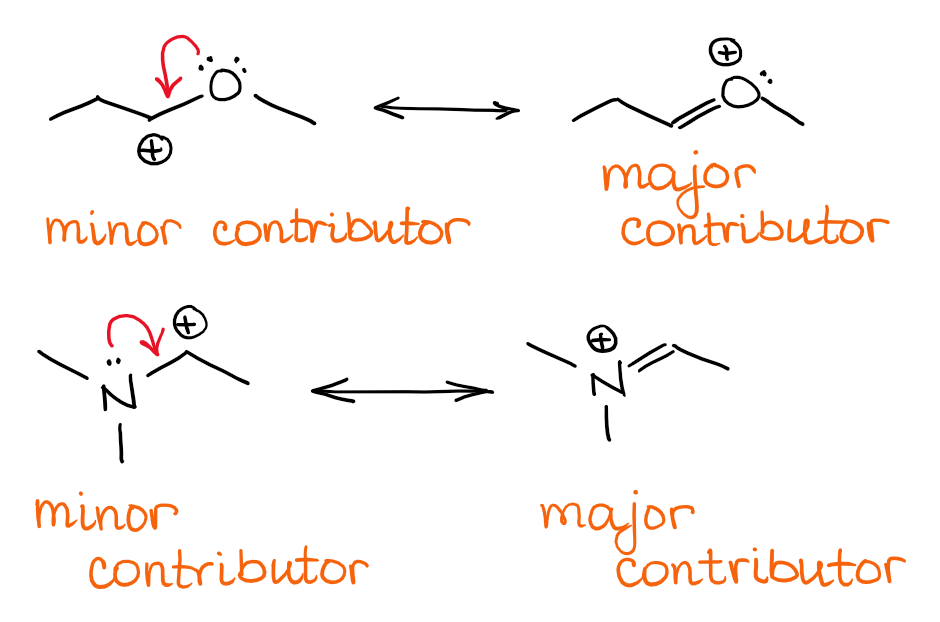
Notice how the structures with the ⊕ on heteroatoms here represent major contributors to the resonance hybrid. When we’re considering different resonance contributors, the structures with full octets will always be the major contributors vs the ones without full octets around all atoms.
Localized vs Delocalized Electron Pairs
The electron pairs that we use in resonance are delocalized. This means that those electrons are not longer located on the orbital of just one atom. Rather, they are spread or shared to some extent between two (or more) atoms. They no longer belong to just one atoms.
Other electron pairs (if any) would be localized. For instance, if we look at the first example with the oxygen, we’ll see that after we use one electron pair on the oxygen for the resonance, the oxygen still has one more electron pair. Those “leftovers” are you localized electrons.
This distinction is rather important because the delocalized electrons will be less likely to participate in any reactions while the localized ones are available. So, when thinking about reactions, we’ll always try to find the localized electron pair and see if that can do some chemistry before moving to delocalized ones or π-bonds and use those electrons.
Empty p-Orbital Interacting with a π-Bond
When the empty orbital is next to a double bond, you can have an interaction between those two making a longer and more stable molecular orbital. The π-bond in organic molecules is made from the p atomic orbitals. This makes the molecular orbital close enough in geometry, so you can have a good orbital overlap between the empty p-orbital of one atom and an adjacent π-bond. Here’s the example:
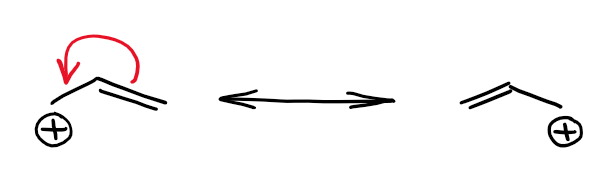
The resonancely-stabilized carbocation above called allylic cation. The important feature of the molecules with this type of a structural motif is that the ⊕ charge is often equally or cole to equally distributed between two or more atoms. This means that there’s no longer a significant lack of electron density anywhere in the molecule. This makes the overall species more stable.
We can represent the orbital interactions in this species with the following orbital picture:
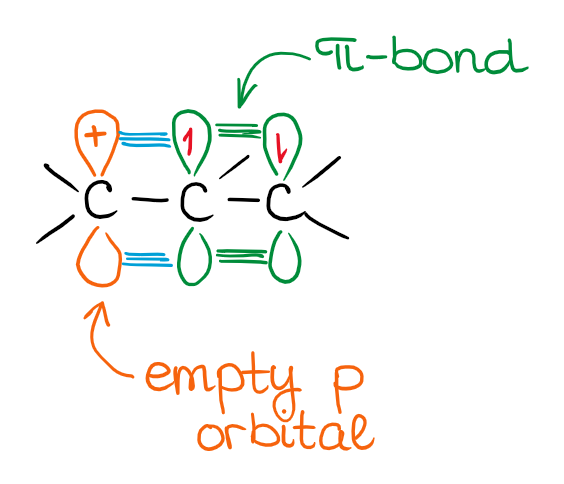
Instead of an empty orbital and a π-bond, you now have a loooong π-style bond spanning 3 carbon atoms. There’s also not going to be a single empty orbital as the electron density is spread through three atoms.
Examples of the Empty p-Orbital Interacting with a π-Bond Type of Resonance
Here are a few example of the allylic systems in different molecules:
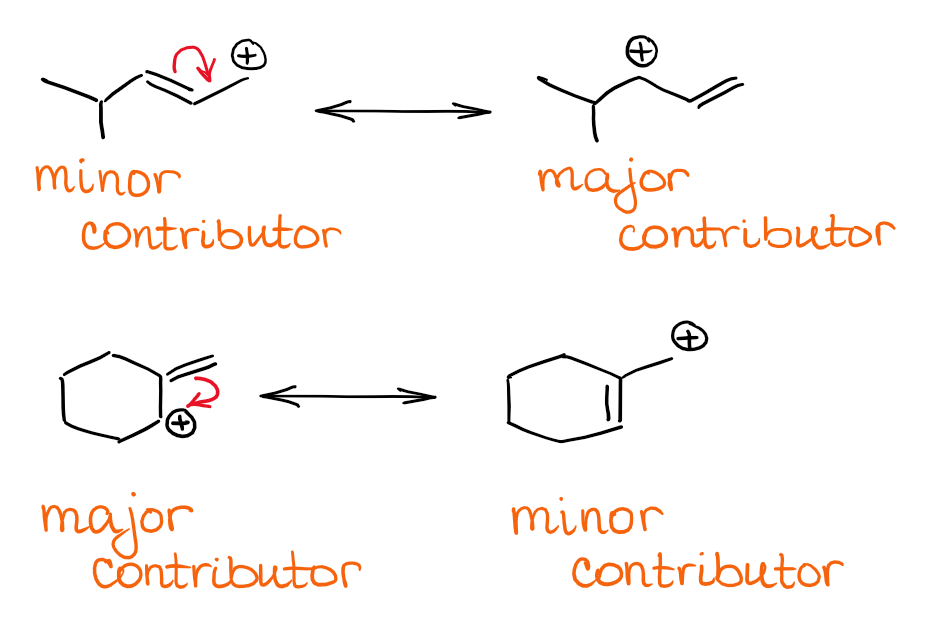
Unless the species is completely symmetrical, you’re still probably going to end up with unequal resonance contributors. In this case, to find the major resonance contributor(s) just look for the position of the ⊕ charge. As the carbocation stability decreases from the 3⁰ to 2⁰ to 1⁰, contributors with 3⁰ cations will be major contributors to the overall hybrid.
Electron Pair Interacting with the π-Bond
When you have either an anion or a heteroatom with a spare electron pair next to a double or a triple bond, you can potentially have the resonance between those. The resulting hybrid may be an anion or a neutral structure depending on the nature of said electron pair. Here’s an example of an allylic anion:
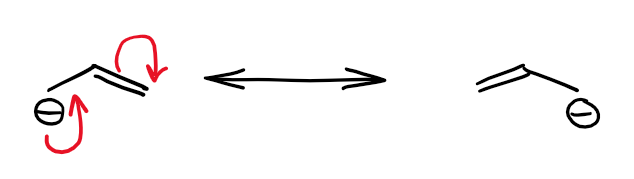
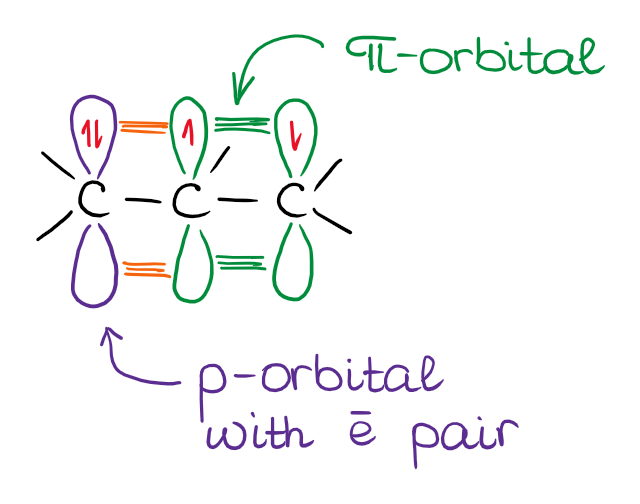
The orbital interaction here is the same as in the case of an empty orbital resonating with the π-bond. However, in this case, the system is electron-rich rather than electron poor.
Examples of the Electron Pair Interacting with the π-Bond
Here are some example for a neutral (heteroatom) and anionic species participating in this type of resonance:
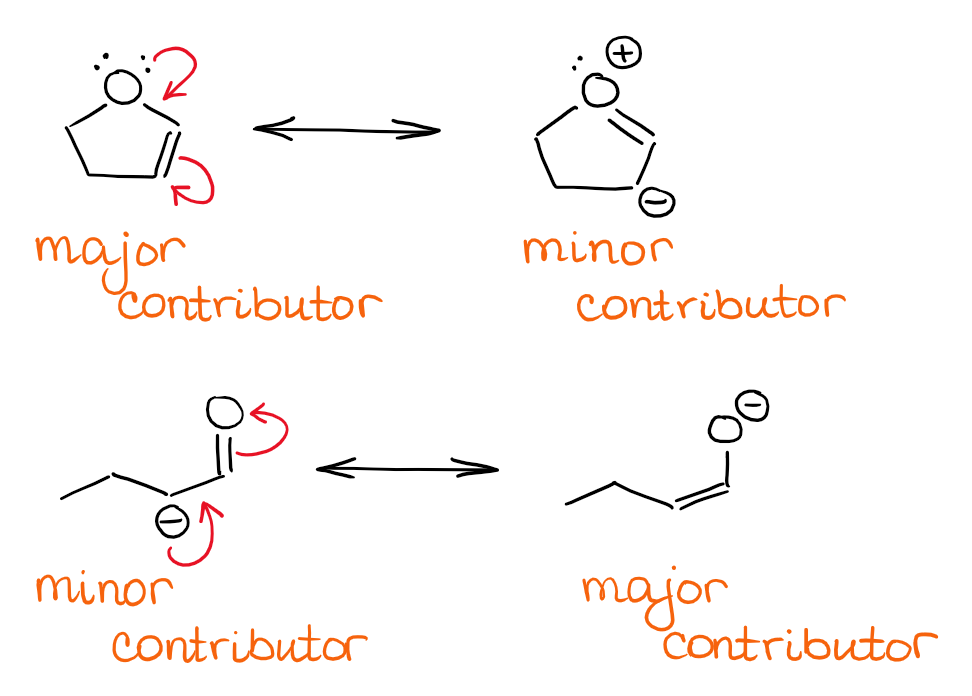
Determining the major and minor contributors in this case might be a little trickier. The neutral contributor is usually the major contributor to resonance when possible. While there are some exceptions from this general rule of thumb (like in the case of amides), it will work for the majority of organic molecules. If, however, you have a negative charge in your system, then the contributor with the ⊖ on the most electronegative atom is the major contributor. For instance, you want to place the negative charge on oxygen rather than a carbon atom in the resonance. This also means that the overall hybrid will have the partial negative (δ-) on the more electronegative atom.
When the ⊖ charge is on the same type of an atom, then you want to check the rest of the molecule for any effects that may stabilize it. The most common, is the presence of the electron-withdrawing groups (EWG’s). Those can stabilize the ⊖ via induction, so having the negative charge closer to an EWG is more favorable.
Interaction of Multiple π-Bonds
This type of resonance stabilization is a hallmark of what we call “conjugated” systems. You would typically see it in polyenes (structures with multiple double bonds) and aromatic compounds. The phenomenon of aromaticity is something we’ll discuss later in this course, but it has its roots in the π-bond to π-bond resonance interactions in cyclic systems.
Here’s an example of the simplest conjugated system with two π-bonds:
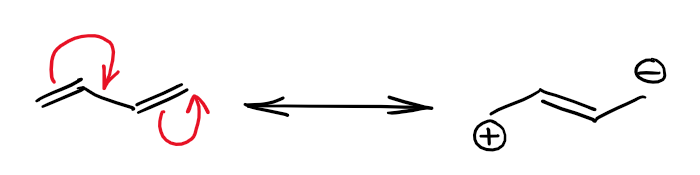
Butadiene (molecule above) is the smallest conjugated diene and the π-bonds interact to make a minor resonance contributor with charges on the outside carbons. The molecular orbital picture for those interactions can be rather complicated, especially so for the larger molecules. Here’s the case of butadiene:
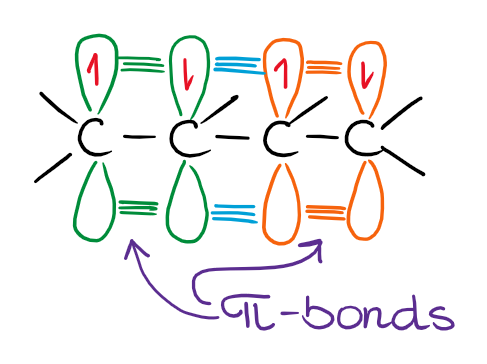
The exact nature of the molecular orbital picture goes beyond the scope of this topic, so we’ll discuss it later. The important part though, is that your double bonds must be able to physically interact by being in the same plane. This would force the entire system to make one long planar structure in 3D.
Examples of π-Bonds Interacting via Resonance
Here are some examples of this type of a resonance system:
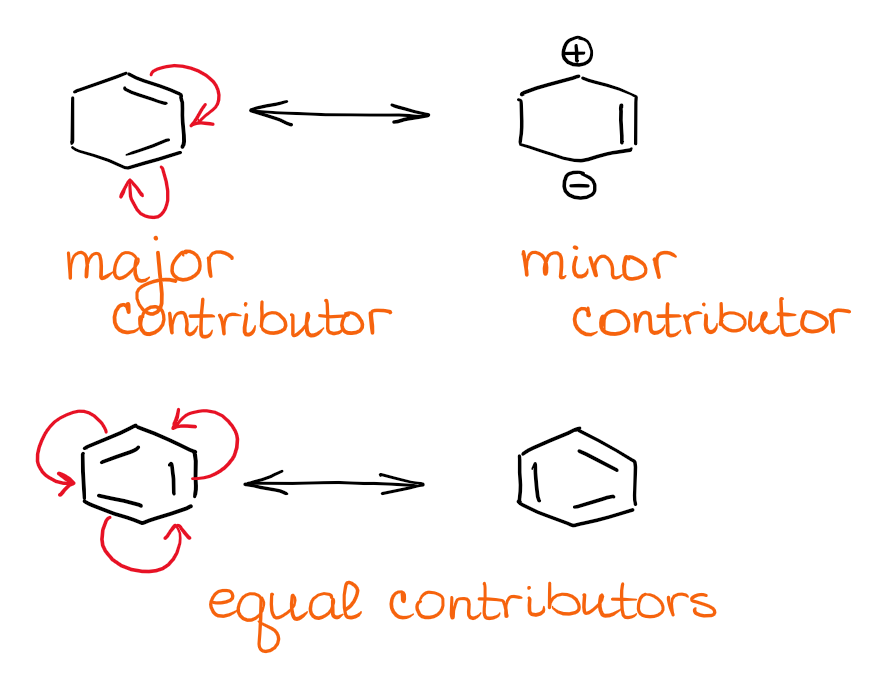
As I’ve mentioned above, the major contributors to the overall hybrid are neutral. This makes it somewhat easier to determine the nature of the hybrid. 😂
Generally, you’re not going to be working with these systems too often. But you still should be able to identify those on the fly so you’ll know there’s a potential complication with their chemistry.
