Nomenclature of Alkanes and Cycloalkanes
Nomenclature of Alkanes and Cycloalkanes
In this tutorial, I want to talk about the fundamentals of the nomenclature of organic molecules. We are going to start with the simplest of the molecules—alkanes. And for the most part I’m going to be focusing on the IUPAC rules in this tutorial. IUPAC stand for the International Union of Pure and Applied Chemistry. Which is an international body that represents chemistry and related sciences and technologies. It’s the world authority on chemical nomenclature among other things. And yes, I totally took this definition off their website.
But before we go into the naming itself, let’s review really quick what the alkanes and cycloalkanes are.
Alkanes are the hydrocarbons that only contain single C-C and C-H bonds. They also don’t have any other functional groups in their structure.
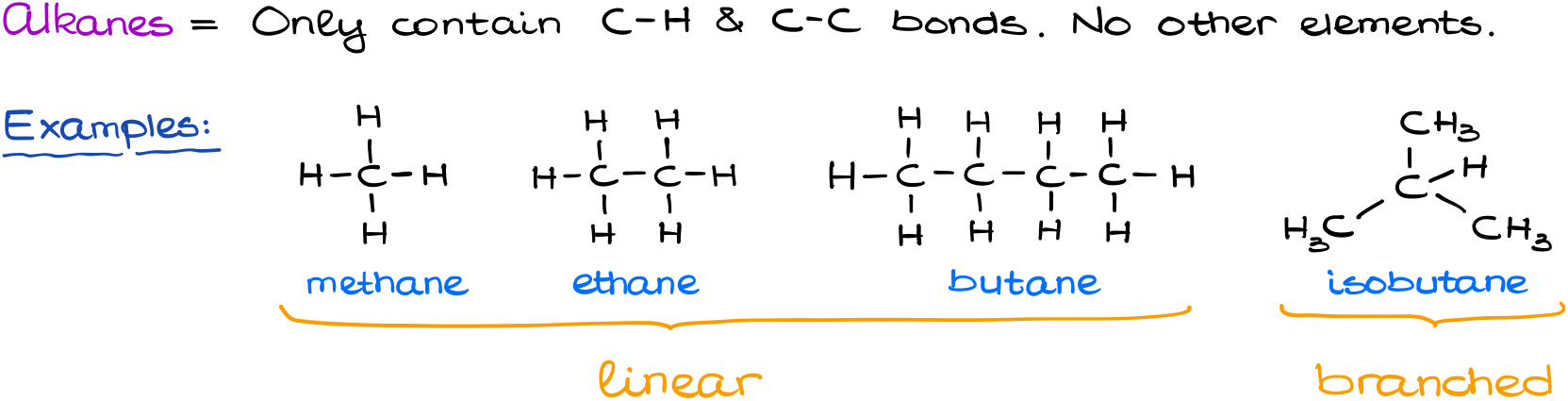
For instance, we can draw a molecule of methane, ethane, butane, and isobutane here. Alkanes can be either linear or branched. But for as long as we have an open-chain molecule with only single C-C and C-H bonds, it’s still an alkane.
In turn, the cycloalkanes are the cyclic versions of alkanes. They also only contain single C-C and C-H bonds. But unlike simple alkanes, which are open-chain molecules, the cycloalkanes have a ring segment in the structure.

For instance, here I have the structures of cyclopropane and cyclopentane.
Parent/Principal Chain Names
In order to start naming molecules, we first need to know the basic building blocks of the nomenclature. Those are the parent or principal names for the chains with the different lengths.
The simplest one contains just one carbon, and we call it methane. For two carbons, we have ethane. Then, for three carbons we have propane. Butane for 4, pentane for 5, hexane for 6, heptane for 7, octane for 8, nonane for 9, and finally, decane for 10.

While there are more names for longer chains, we’ll limit ourselves to just the first ten examples in this tutorial. Also, in my experience, most instructors will only require you to learn the first ten anyways. And yes, you’ll have to memorize those. There’s no other way around it.
What happens if you need to know the names beyond the first ten, like undecane for C11, dodecane for C12, or eicosane for C20, well, google it. It’s a rare case when you’ll actually need to know anything beyond the first ten. If your instructor does indeed require you to know beyond that, come back to this tutorial and comment “Victor, you’re full of shit, my instructor asked for dotetracontane on the exam!” which is, by the way, C42H86.
But just in case, here’s the next ten.
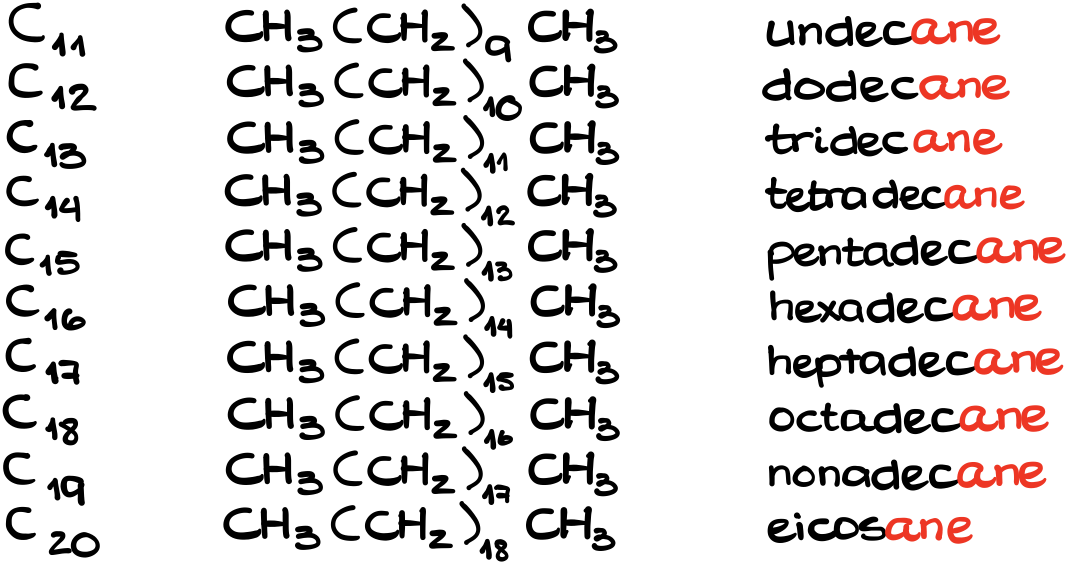
Anyways, back to reasonable chemistry here.
All these as you can see, are all open chain examples. So, how do we name the cyclic alkanes?
Well, it’s very similar, actually.
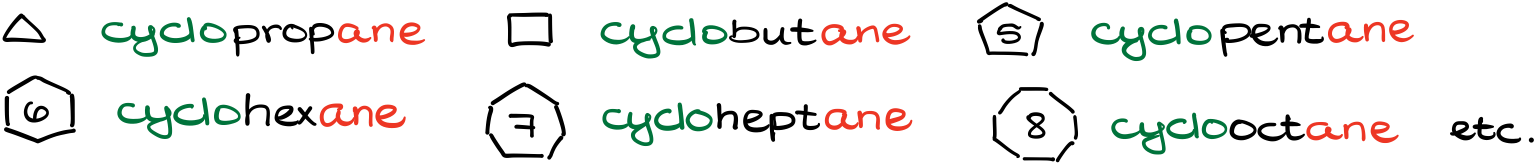
For the cycloalkanes, you’ll just add the prefix “cyclo-” to the name which signifies that this is a ring. For instance, a three-membered ring is a cyclopropane, a six-membered ring is cyclohexane, etc.
Notice, how all these molecules have the ending “-ane.”
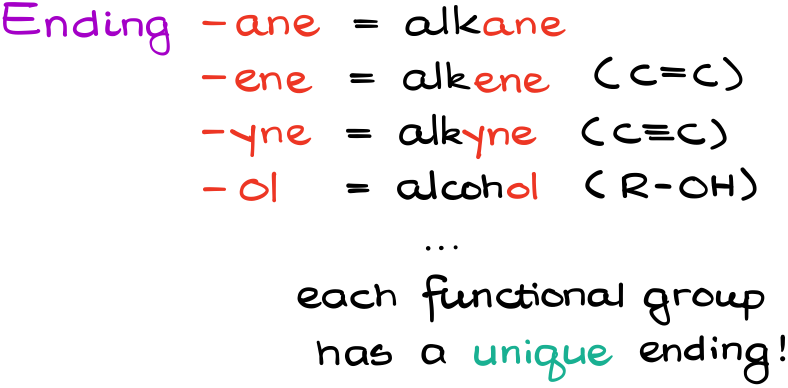
This is specifically the ending for alkanes. So, whenever you see a name that ends with the “-ane,” you automatically know that you’re dealing with a molecule that only contains single bonds.
Different functional groups have different unique endings. For instance, molecules with C=C bond have the ending “-ene,” while alcohols end with “-ol” etc. You’ll learn about all of those later in the course, so don’t worry about those for right now. The purpose of this tutorial is to build a foundation that we’ll use in the future to add new layers of the nomenclature complexity as we cover more chemistry along the way.
I actually suggest you copy these names down as you’ll need them for the rest of the tutorial. As I’ve mentioned, you’ll have to memorize these names.
You also may want to make flash cards with those to make sure you do memorize these names before your first test. I have never seen an instructor allowing you to bring or giving you the nomenclature “cheat sheet” on the test. However, I can guarantee you are going to see nomenclature on your first organic chemistry test. Unless, of course, your instructor hates it and decides to skip it, which happens once in a blue moon. But even if that’s the case, you’ll still need to know the nomenclature for your MCAT, DAT, ACS, and other tests you may be looking to take in the future.
Naming Substituents
Now, as I’ve mentioned earlier, our molecules can be branched.
In reality, most organic molecules are not just simple chains and cycles. They contain all kinds of side chains and branches coming off the main stem. We often call those branches “substituents” so get used to hearing this term as well. I’ll be using the terms “branch” and “substituent” synonymously and interchangeably in this tutorial.
So, how do we call those branches?
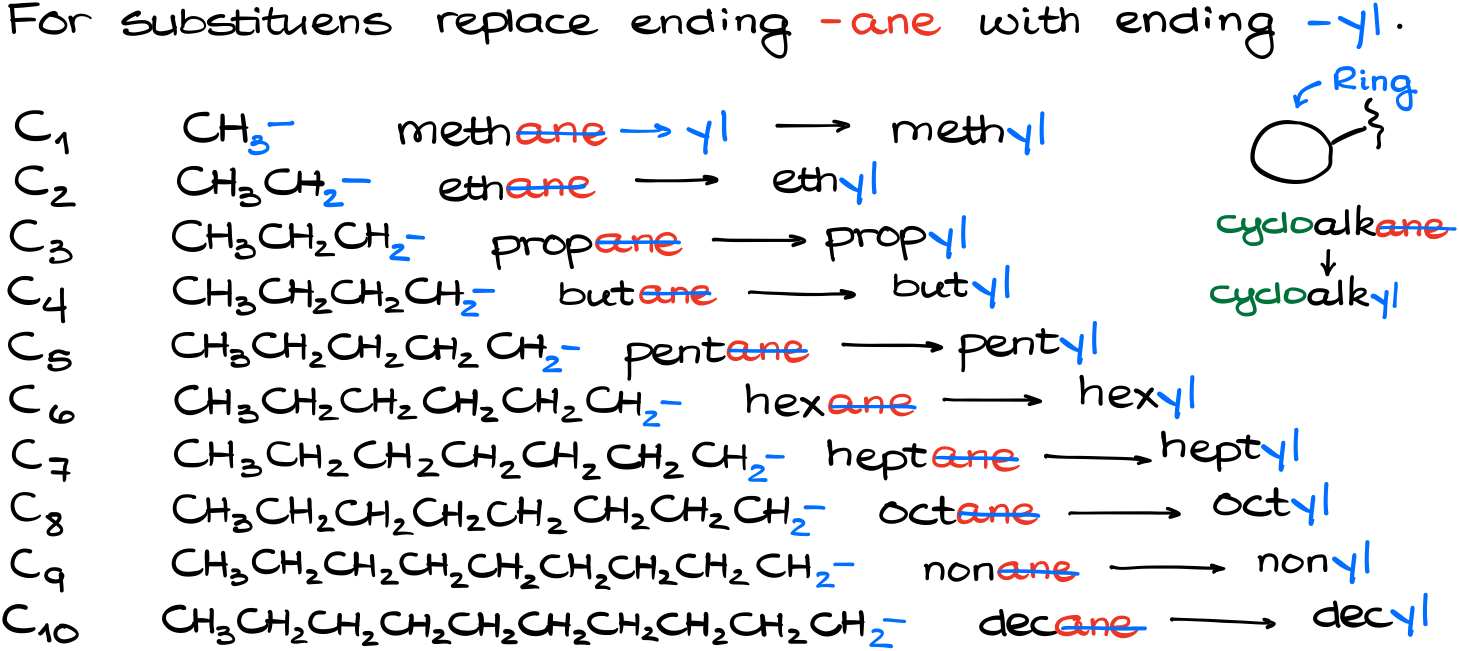
We’re going to construct the substituent’s name by replacing the ending -ane with the ending -yl in the parent’s name. For instance, if the substituent only has 1 carbon, aka a CH3- group, we’ll call it a methyl group.
And by the way, it works exactly the same way if we have a cycle as a substituent. Instead of saying cycloalkane we’ll say cycloalkyl.
Examples
Now, when we know the building blocks, let’s go over the actual nomenclature rules and look at a few examples.
First, we’ll start with this molecule over here. And btw, for the rest of this tutorial, I’m going to be using the skeletal, or line structures. So, if you’re not very comfortable with those, I suggest you pause this tutorial and go review what the bond-line structures are all about. I also have some tutorials on this topic you may wanna check out.
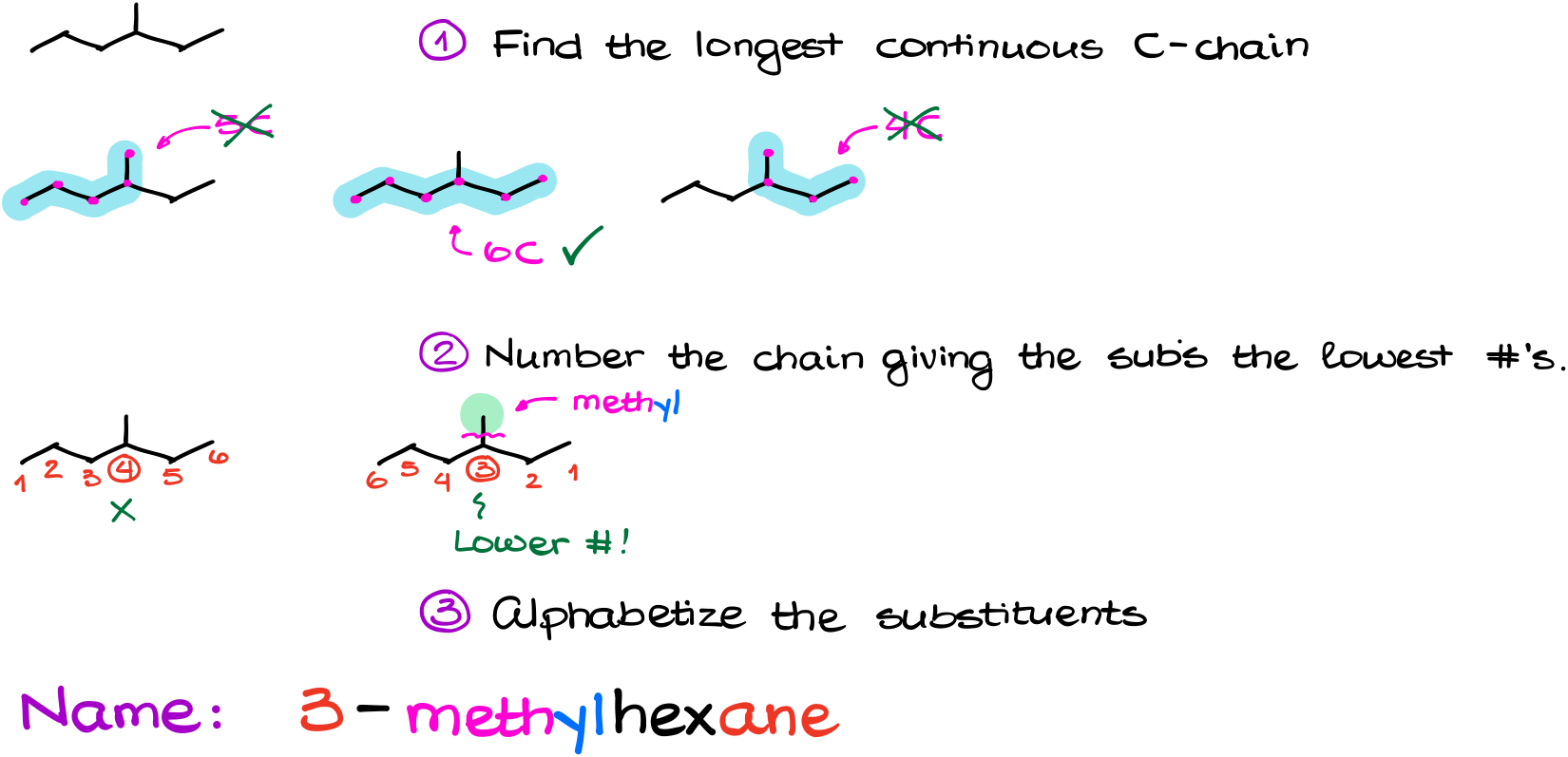
So, the rule #1 is to find the longest continuous carbon chain. For this example, we can have a 5-carbon, a 6-carbon, and a 4-carbon chains. And as we’re looking for the longest one, we’re going to choose the 6-carbon chain.
Next, we need to number our chain in such a way as to give our substituents the lowest possible numbers. Here, we can number our chain in two different ways. And since we’re looking for the numbering system that would give us the lowest possible number at our substituents, the one on the right is the one we’re going to choose here.
Finally, we’re going to alphabetize our substituents and append them to the principal chain’s name. In this example we only have a methyl group, so nothing really to alphabetize.
Thus, our name is 3-methylhexane. Notice, that I have to specify the location of my substituent. Also, when writing your name, we will separate the numbers from letters by dashes and if we have multiple numbers, we’ll separate them by commas. Do NOT put any spaces or any other punctuation marks in your name. Later on, you’ll learn about more advanced rules that indeed use other punctuation marks, brackets, spaces, etc. But for now, we only know the dash between numbers and letters and comas between numbers. That’s all.
So, to summarize the rules, we have:
- Find the longest chain;
- Number the chain to give the lowest numbers to substituents;
- Alphabetize the groups in the name.

Here’s another example.
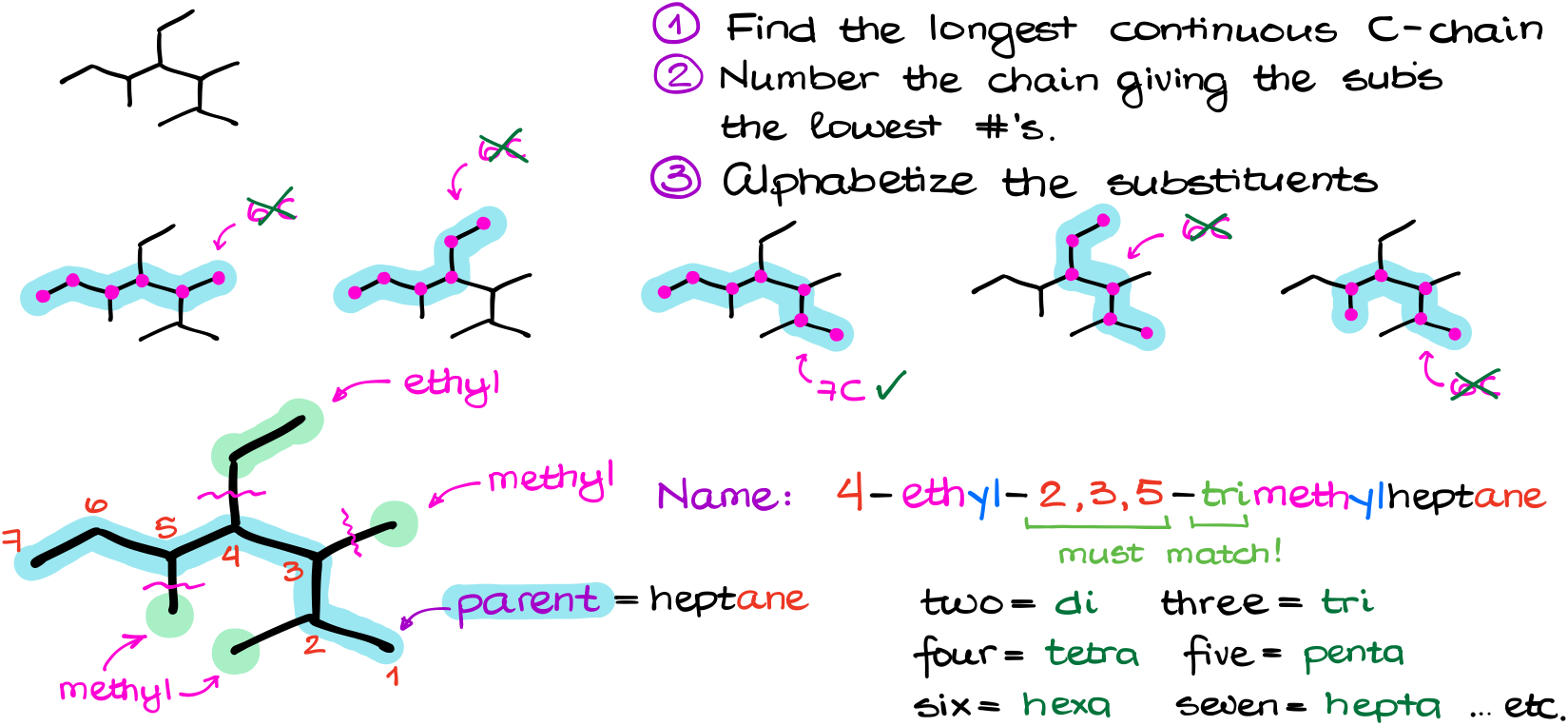
First, we’re looking for the longest chain. We’ll have a few options. The list in the figure above is far from exhaustive, but I didn’t want to waste time drawing all possible options. We can clearly see that the longest chain here contains 7 carbons. Notice, your longest chain does not have to be the horizontal one. It can twist and turn in any number of ways. So, don’t just assume the horizontal chain is your parent.
Next, I’ll identify my substituents and number my chain. For the substituents I have a few methyl groups and an ethyl group. Be very careful not to include the parent chain carbons in your carbon count for the substituents. It’s a good idea to carefully circle your parent chain and show an attachment with a fine squiggly line like I did here to resist the temptation.
In terms of numbering, starting from the right will give me the lowest possible numbers.
Finally, alphabetizing my groups and adding them to my principal chain’s name, we get 4-ethyl-2,3,5-trimethylheptane.
Here’s something important I want to point out with this example. We have several methyl groups, so we must say where exactly those methyl groups are. This is what those “2,3,5” numbers are all about. Also, we have three of those, so we must state that by using the “tri” prefix. This is a very important rule and one of the most common mistakes: your numbers (we also call them locants) must match the numeric prefix for how many groups you have.

So, we’re going to add a few pieces to our memorization list. You may wanna copy them down as well.
Ok, here’s another example.
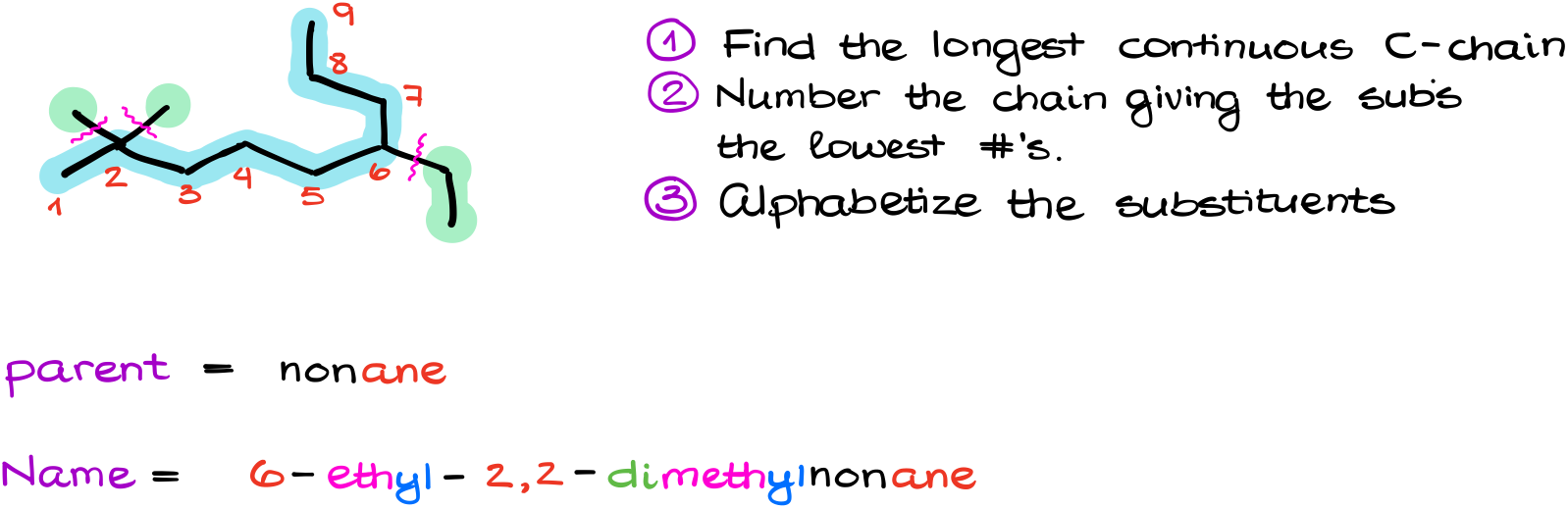
Following our steps, we can see that the longest chain contains 9 carbon atoms. This means the parent’s name is nonane.
Next, we’ll number this molecule from left to right as this will give us the smallest possible numbers.
Looking at my substituents, I see that I have a couple of methyl groups and an ethyl group. So, putting it all together in the alphabetic order gives me 6-ethyl-2,2-dimethylnonane.
A couple of important points I want to make here as well.
First, the prefix “di” is not the part of the group’s name, so we are not going to count it for the alphabetical order. So, we’re still going to name ethyl group before the methyls.
Second, although both methyl groups are sitting on the same carbon, we still need to specify this location twice. That’s why we say “2,2” here for our locants. It might seem redundant at first but think about it as the address for the group. And since each group must have its own address, we are going to say it twice.
Nomenclature of Complex Substituents
Now, up to this point, all our substituents were simple one- or two-carbon chains. This is, obviously, not what we are going to see all the time. Occasionally, we are going to see some more complicated groups sitting on our parent molecule. We call those “complex substituents” and they have their own nomenclature rules.
For the two simplest substituents, methyl and ethyl group, we can’t have any variations.
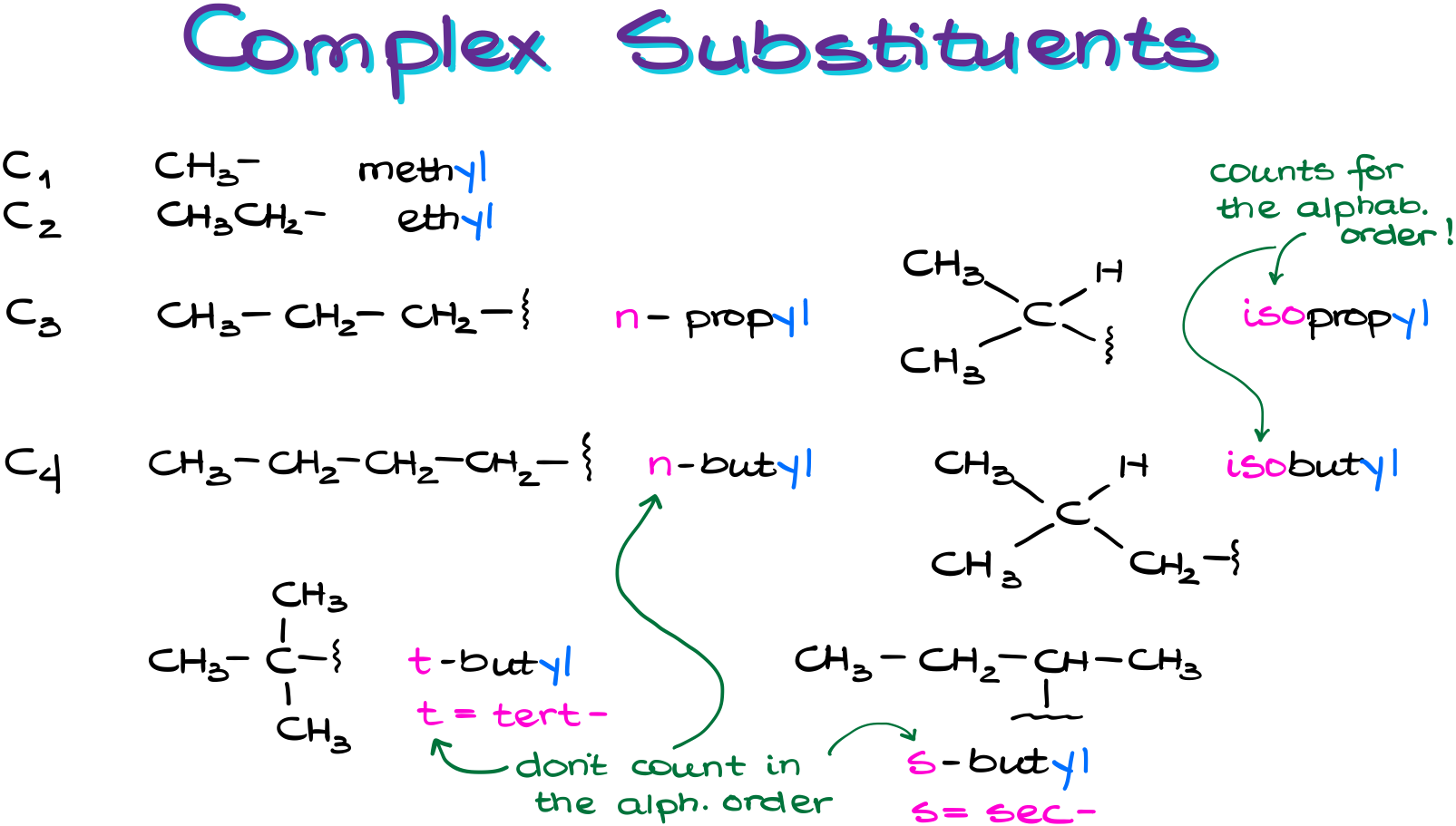
However, starting from 3-carbon substituents, we can have variations. For instance, the propyl group can attach to the parent in a linear fashion. This is called n-propyl, where “n” stands for “normal” or linear. Or alternatively, the propyl group can attach to the parent via the middle atom. In this case we are going to call it an isopropyl group. Here “iso” means an “isomeric” group.
A 4-carbon substituent chain has even more variations. We have a normal butyl, isobutyl, tert-butyl, and sec-butyl. “Tert” stand for tertiary and “sec” stands for secondary. BTW, when you’re typing the names, the “sec” and “tert” must be italicized.
And guess what? You have to memorize those names as well. These are the common names. And the IUPAC allows us to use the common names for the C3 and C4 substituents. Those are so-called “retained” names, so we can freely use them instead of the “correct” ones. If we have anything longer than that, we’ll have to use the complex substituent nomenclature.
We’re going to talk about the complex substituents in more details in another tutorial, so for now we’ll just limit ourselves to these as they are allowed by the IUPAC.
Also, another bit you have to memorize is that the prefix “iso” is counted towards the alphabetization while the prefixes “tert” and “sec” are not. So, for the alphabetic order, the isobutyl group starts with “i” while the tert-butyl starts with “b” as the “tert” part doesn’t count.
I know there’s a lot of memorization here, but learning nomenclature is like learning a different language: you gotta memorize the vocabulary. So, there’s nothing we can do about it.
Examples
Now, how does that look like in an actual molecule?
For instance, let’s look at this molecule.
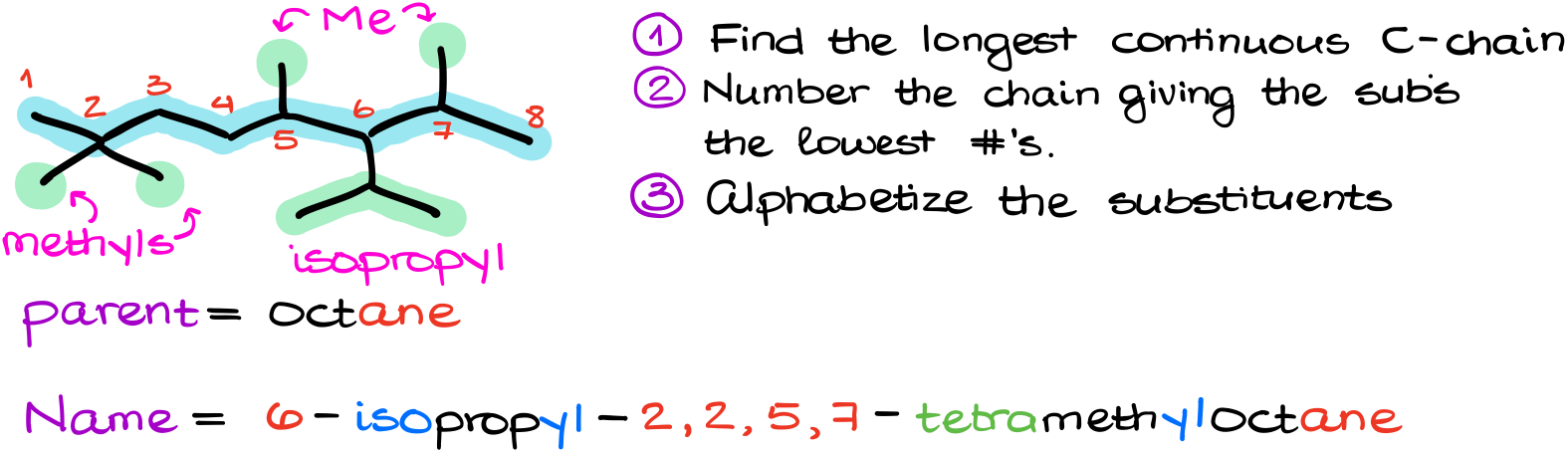
Following our rules, we’ll find the longest chain and number it.
Next, I’ll identify my groups. I have four methyl groups and one isopropyl group in this molecule.
And finally, alphabetizing my groups, I get 6-isopropyl-2,2,5,7-tetramethyloctane. See, since the prefix “iso” is counted for the alphabet, the isopropyl group has to come before the methyl groups in the name.
Or, for instance this example.
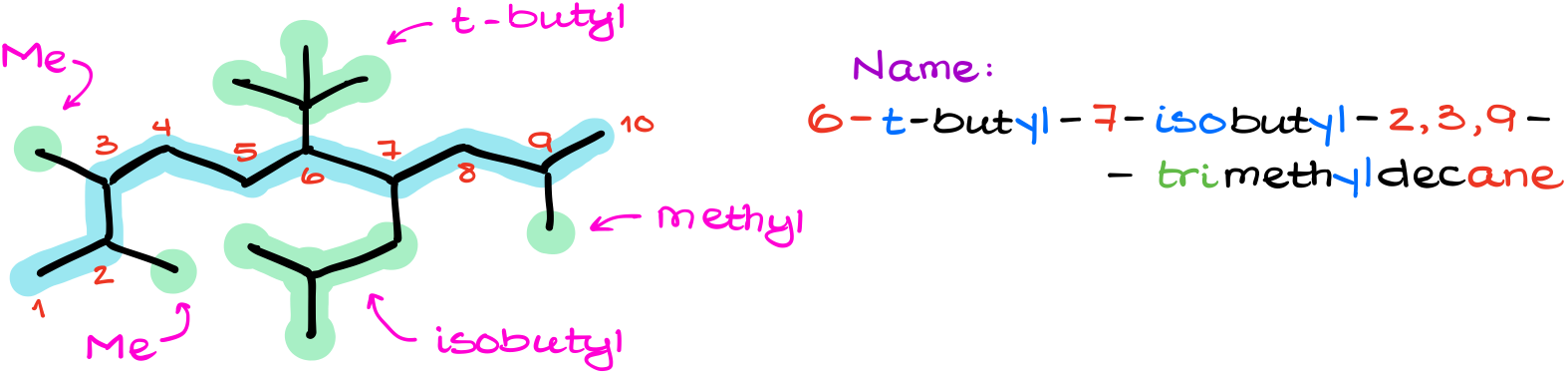
Here, we have the longest carbon chain containing 10 carbons. We also have two different 4-carbon substituents: t-butyl and isobutyl. We also have a few methyl groups too. Which, after proper alphabetization of our groups gives us 6-t-butyl-7-isobutyl-2,3,9-trimethyldecane.
Nomenclature of Cycloalkanes
Alright, let’s now check out the nomenclature of cycloalkanes. The rules are very similar.
For instance, this molecule has 6 carbons, so we’ll call it cyclohexane.

But what if I add a couple of groups to it?
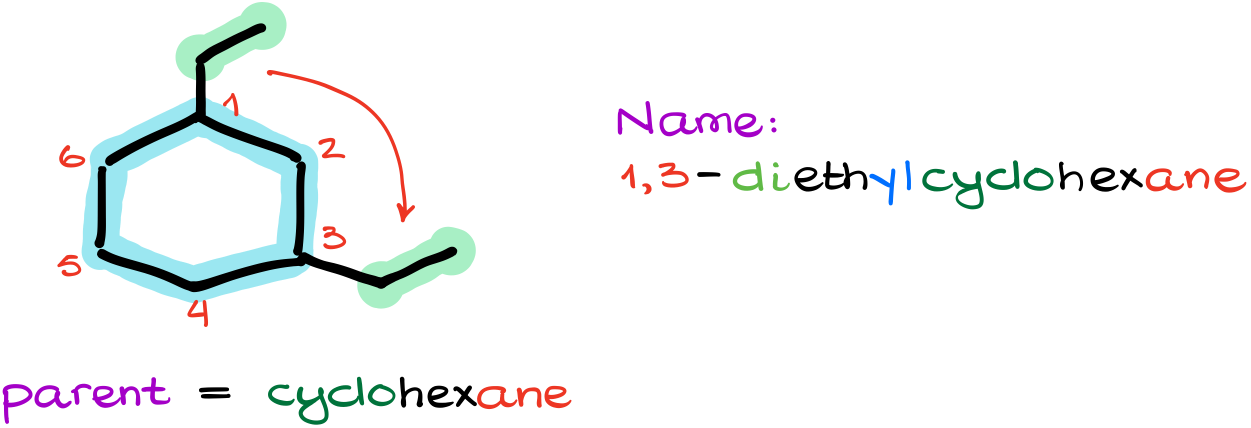
Well, my parent is still a cyclohexane. But where are we going to start our numbering?
So, since cycles don’t have the “beginning” or the “end,” we are going to number from the place where our first substituent is and then we’ll go towards the next closest substituent. In this case, I’ll start numbering from the top carbon where I have an ethyl group and will go clockwise towards the other ethyl group. In this particular case, it doesn’t matter which group I start numbering from as both ways will give me the same locants for my substituents.
Thus, my name for this molecule is going to be 1,3-diethylcyclohexane.
What if we have different groups?
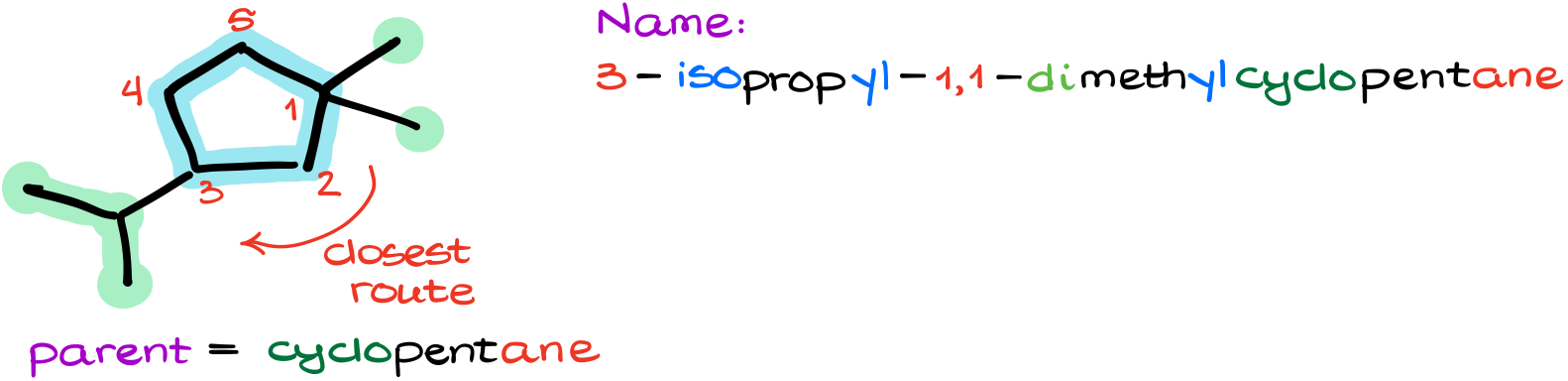
We still need to number our molecule in such a way as to give the lowest possible numbers. Thus, we’ll start numbering from the carbon where we have two methyl groups and continue towards the isopropyl group. This gives us 3-isopropyl-1,1-dimethylcyclopentane.
So, it doesn’t matter how big or ugly the molecule is. For as long as you follow these rules, you’ll be able to name anything (or almost anything till you learn more rules). And even though we are going to be adding more rules and increasing the molecule complexity, the fundamentals are still going to be the same.
Tiebreakers
Now, occasionally you’re going to face the molecule where our rules can give two (or more) possible names. Since the IUPAC does not allow for ambiguity or multiple names, there are tiebreakers.
The firs one is when you have two possible longest chains.
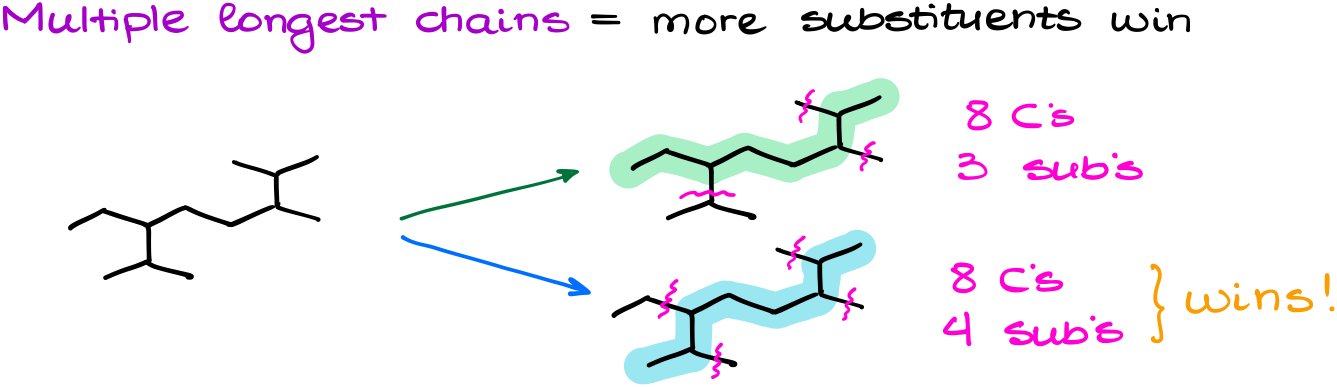
In cases like this one, we need to see how many substituents we have on each of the chains. The more branches you have going off the parent, the more important that chain is going to be. So, in this example, I have a situation in which I have 8 carbons in each possible chain. But the bottom one has 4 branches, while the top one only has 3 branches. And as the bottom one has more substituents, it wins the tie for the longest chain.
And another one you’re likely to encounter is when you have multiple different ways of numbering your parent chain.
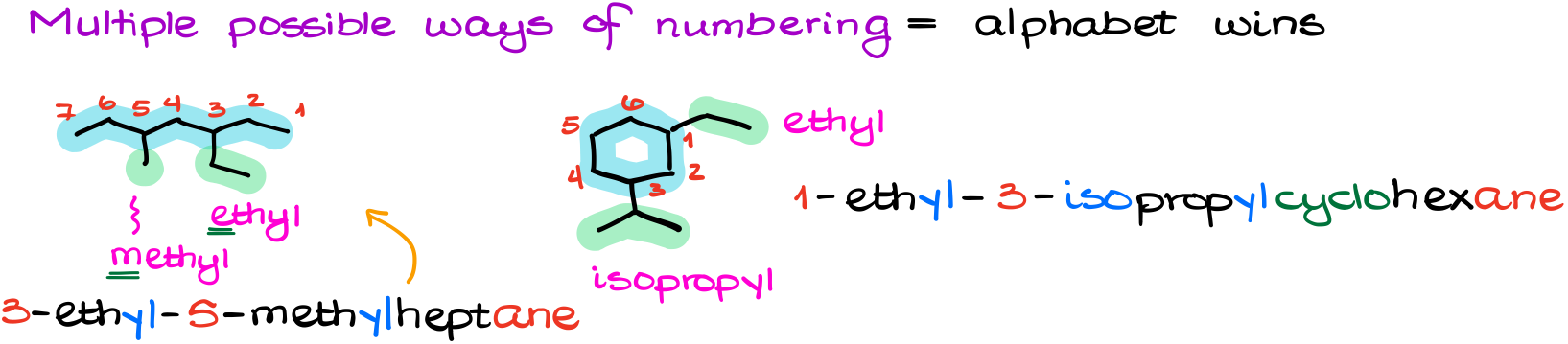
In this case, the substituents that are earlier in the alphabetic order will take the precedence and will have to have lower numbers.
For instance, here I have a couple of examples. One, an open chain molecule in which we have a competition between the ethyl and a methyl group. Due to the alphabetic order, the ethyl group gets #3. In the second example I have the competition between the isopropyl and the ethyl groups. Again, the ethyl wins here getting #1.
So, remember, if you can’t find any difference, the alphabet is going to be your last resort. And this is also something very important to remember: do NOT check for the alphabet till you’ve exhausted all other tiebreakers.
Is your head spinning from all the rules and details? I bet it does. But don’t worry, it gets easier with practice. Like when you’re learning a new language, at the beginning you have to think about every word and every grammar rule. But as you practice more and more, the words and constructs start coming easily and naturally. The organic nomenclature is kinda like that. So, make sure you do a lot of practice.
