Nomenclature of Alkynes
In this tutorial, I wanna talk about the nomenclature of alkynes.
Alkynes are unsaturated hydrocarbons with at least one carbon-to-carbon triple bond. They’re a fundamental part of organic chemistry and show up all over the place in health sciences and pre-med studies. That’s because they’re biologically relevant, highly versatile in synthetic chemistry, and just generally useful. And yes, you’ll definitely need to know how to name them for your test.
That’s exactly what we’re going to cover here: how to name alkynes and cycloalkynes, along with the edge cases that tend to trip students up on exams. So grab your coffee, open up your notebook to work through the examples with me, hit that LIKE button for good luck on the test, and let’s dive in.
Fundamentals of the Alkyne Nomenclature
As a functional group, alkynes are named with the suffix “-yne.” Just like with any other hydrocarbon, we start by finding the longest continuous carbon chain that includes the triple bond. For instance, in the first example, we count nine carbons in the main chain. The next step is to number that chain in a way that gives the lowest possible number to the first carbon of the triple bond.
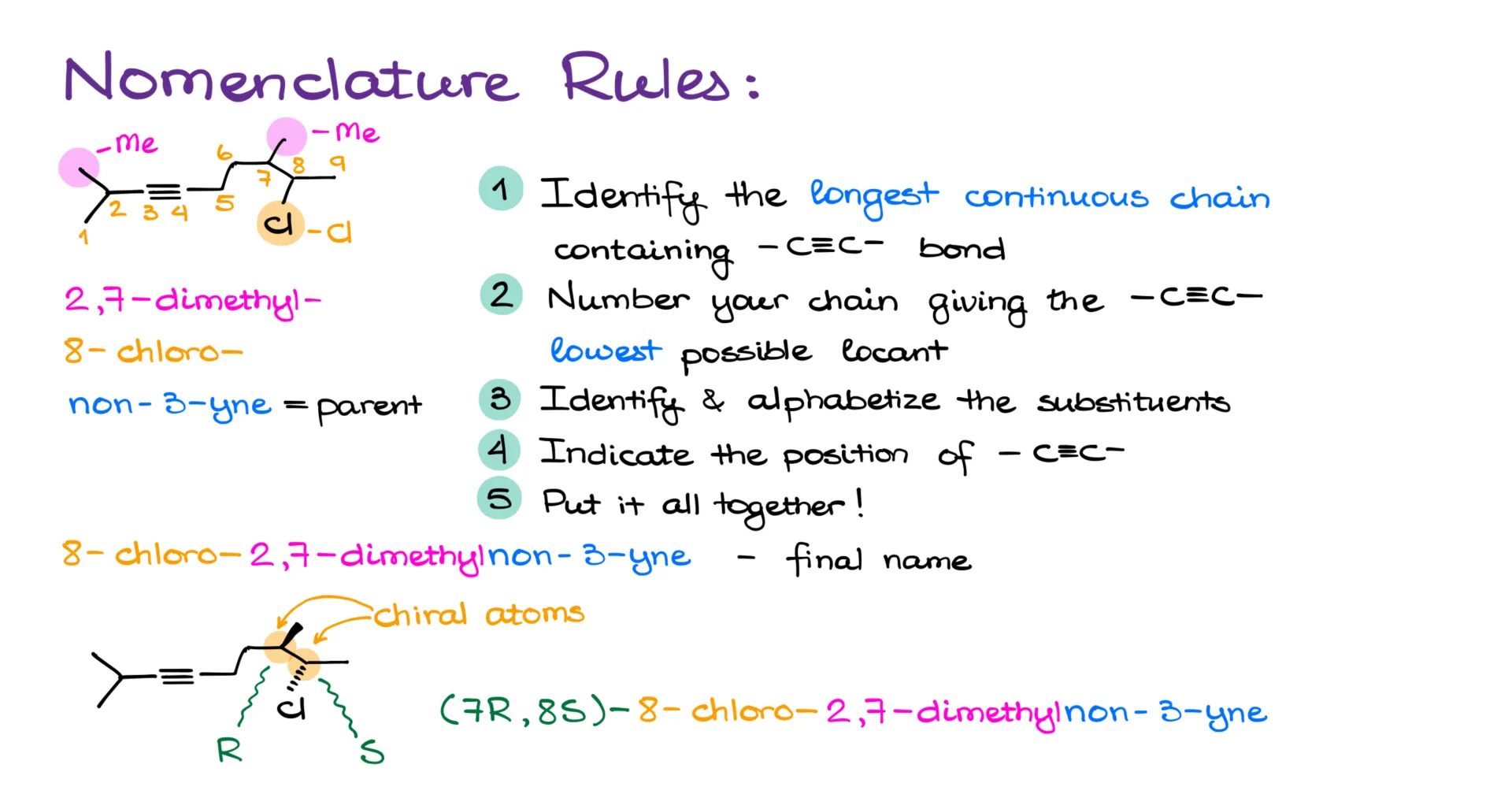
In this case, numbering from the left puts the triple bond starting at carbon 3. If we tried it from the right, we’d hit carbon 6 first, which is obviously higher. And if there’s a tie in numbering the triple bond, we go to the usual tiebreakers—first checking the positions of substituent groups, and if still tied, we use alphabetical order.
Once the chain is numbered, we identify and alphabetize the substituents. Here, we have two methyl groups on carbons 2 and 7. That makes it 2,7-dimethyl. A quick reminder: prefixes like “di-” don’t count in the alphabetical order—only the first letter of the group name does, so “methyl” gets ranked by “m.”
We also have a chlorine on carbon 8, so that gives us 8-chloro. In the final name, substituents are always listed alphabetically, regardless of their positions. I know it doesn’t always feel logical, but it’s what the rules say, so we roll with it.
Next, we mark the position of the triple bond within the parent chain. Since it starts at carbon 3, we name the parent chain as non-3-yne.
Putting it all together, the full name is 8-chloro-2,7-dimethylnon-3-yne.
Now, if we had any stereochemistry involved—say, if there are chiral centers—we would include stereodescriptors at the beginning of the name, just like for other organic molecules. So if we add stereochemistry at the chiral carbons here and assign R/S descriptors, let’s say the methyl-bearing carbon is R and the chlorine-bearing carbon is S. That gives us (7R,8S)-8-chloro-2,7-dimethylnon-3-yne.
And listen, make sure you know how to assign these descriptors. I can’t tell you how many students come out of exams telling me they lost points just because they forgot about stereochemistry. I swear, if I had a quarter for every time that happened, I’d have my mortgage paid off by now. But anyway…
Examples
Let’s take a look at another example.
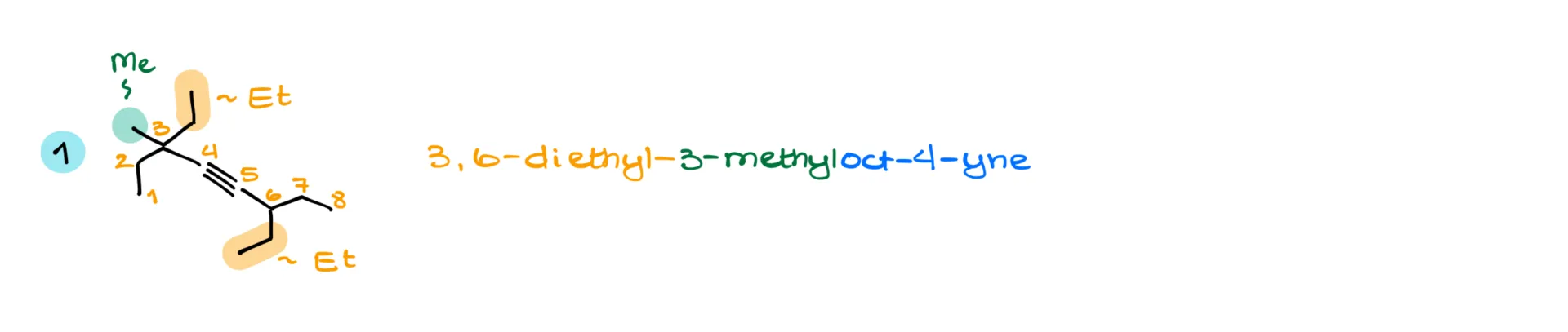
In this one, we’ve got eight carbons in the longest chain. When numbering, you’ll notice that whether we go from the left or right, the triple bond ends up starting at carbon 4 either way. So, we go to our tiebreakers. Numbering from the left gives us two substituents at carbon 3, while from the right we get one on carbon 3 and two on carbon 6. Since we want the lowest positions for our groups, left-side numbering wins.
The substituents here are two ethyl groups on carbons 3 and 6, and one methyl group also on carbon 3. That makes it 3,6-diethyl-3-methyloct-4-yne. This molecule doesn’t have any chiral centers, so no need for stereochemistry here.
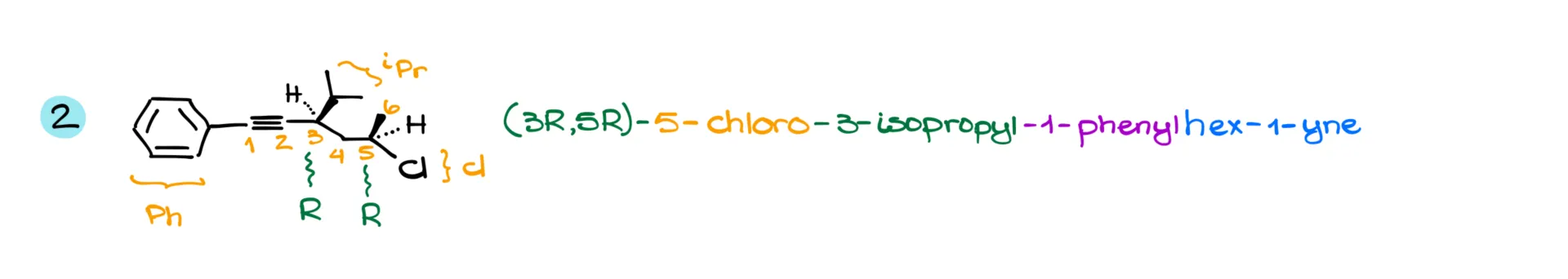
Now, the next example does include stereochemistry. We’ve got six carbons in the longest chain, and since the aromatic ring is attached at position 1, we’re going to start numbering there—you can’t get any lower than 1, after all.
We also have an isopropyl and a chlorine substituent. Just a heads-up: the “iso” in “isopropyl” does count for alphabetical purposes.
So, we put the name together as 5-chloro-3-isopropyl-1-phenylhex-1-yne. Since we have chiral centers at positions 3 and 5, and both are R, the full name becomes (3R,5R)-5-chloro-3-isopropyl-1-phenylhex-1-yne.
Now, what about cyclic alkynes?

Just like with any other cyclic molecule, we number the ring starting from the functional group—in this case, the triple bond—so that it gets the lowest possible number. You always, and I mean always, number through the triple bond, not around it. From there, we keep numbering to give the lowest locants to any substituents. In our case, we number clockwise to make that happen.
So, the final name for this molecule is 4,4-dichloro-7,7-dimethylundecyne. Notice that we don’t specify where the triple bond is—because in cyclic molecules, numbering starts from it by default. Also, since there’s no stereochemistry, we don’t need to mention any descriptors.
One last note before we move on: locants always go directly before the functional group. This is the current IUPAC Preferred IUPAC Name format (PIN). You might still see older names where the locant comes before the parent chain name, but that’s outdated and no longer recommended.
Alkenes vs Alkynes (When Both Are Present)
Now, here comes the tricky part: what if there’s both a double and a triple bond in the same molecule?
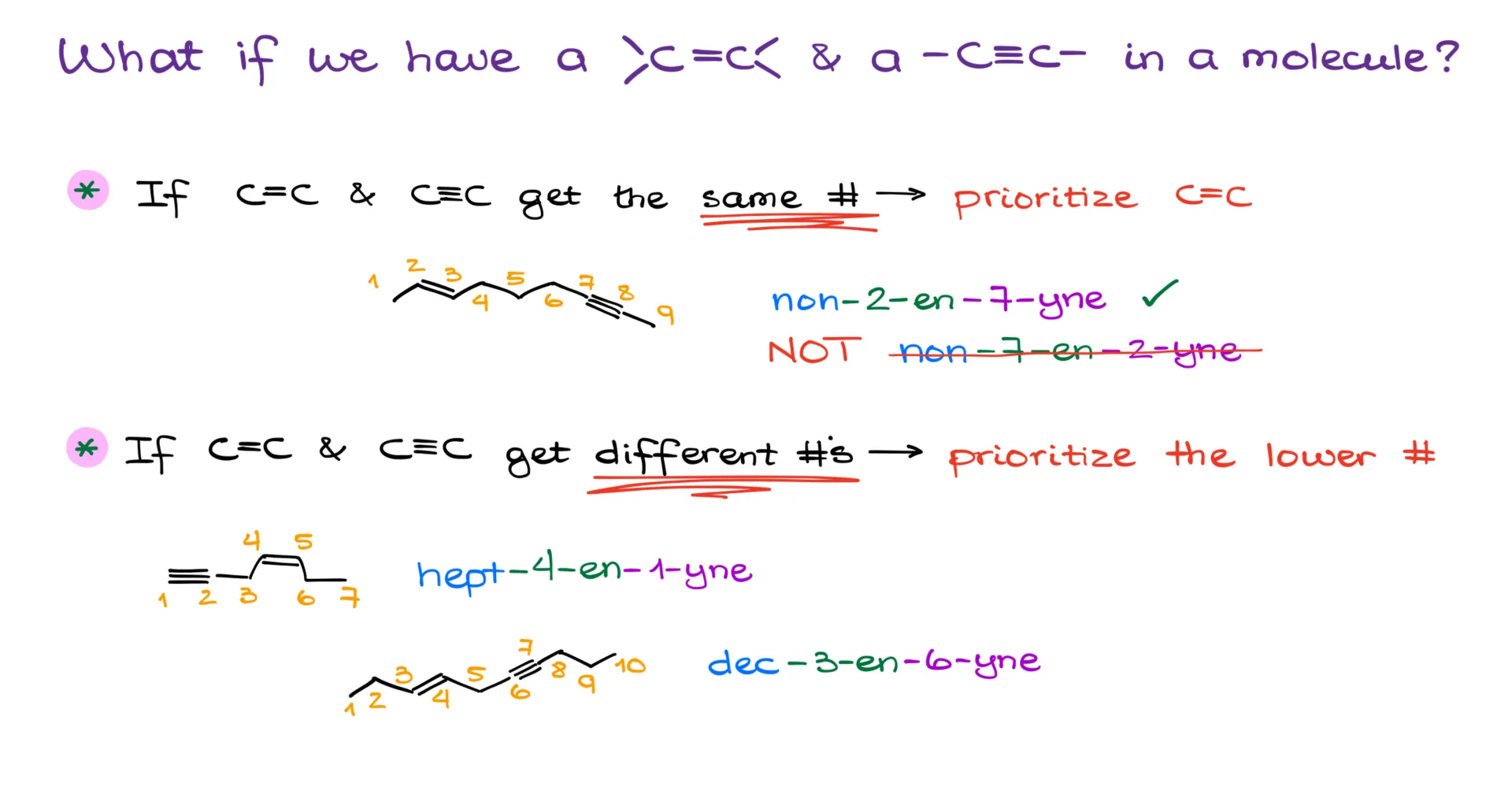
Good news: IUPAC has clear rules for that. Bad news: those rules have changed a few times over the last few decades, and not all instructors are caught up. So I’ll tell you what the 2013 IUPAC guidelines say, but you should double check with your instructor. After all, they’re the ones grading you. That said, standardized exams like the ACS and MCAT do use the rules I’m about to explain.
If the double and triple bonds end up with the same number, the double bond takes priority. So, for example, the correct name would be non-2-en-7-yne, not non-7-en-2-yne.
If numbering from one end gives the double bond a lower number and the triple bond a higher one—or vice versa—then we go with the option that gives the lowest number to either.
So you’d get names like hept-4-en-1-yne or dec-3-en-6-yne, depending on the structure.
And that wraps it up.
