Even Wolverine’s Claws Would Break From This Adamantane Mechanism
In this tutorial, I wanna talk about this interesting two-step reaction that, at first glance, looks like a simple Williamson ether synthesis. We start with an alcohol, treat it with sodium hydride, and then add an alkyl halide. So naturally, we’d expect to form the corresponding ether.
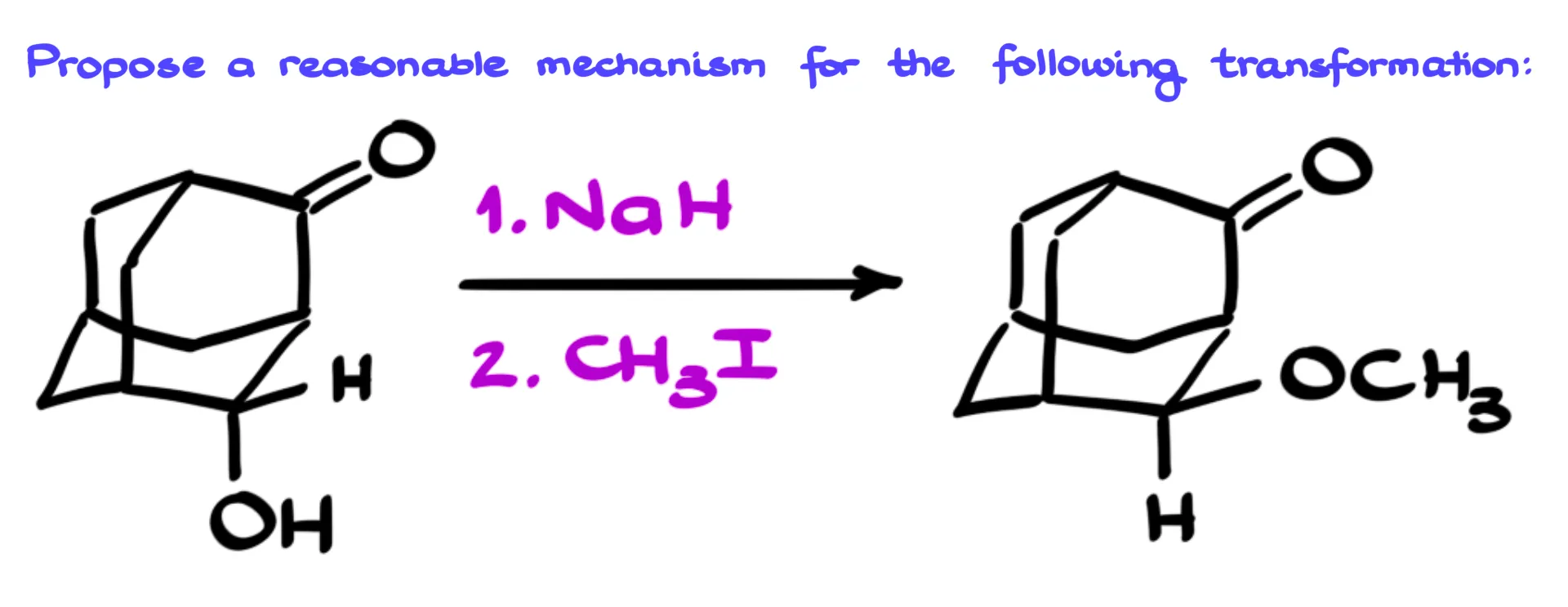
But there’s a twist — the carbon that originally held the OH group suddenly flips its stereochemical configuration. That’s strange because we know this kind of inversion doesn’t normally happen in a Williamson ether synthesis. So let’s take a closer look and see what’s really going on here.
Mechanism
Let’s start by redrawing our starting material. The first step in the mechanism will be an acid–base reaction. Sodium hydride, Na⁺H⁻, is a very strong base but not a nucleophile. So the first reasonable step is simply deprotonation of the alcohol, forming the corresponding alkoxide.
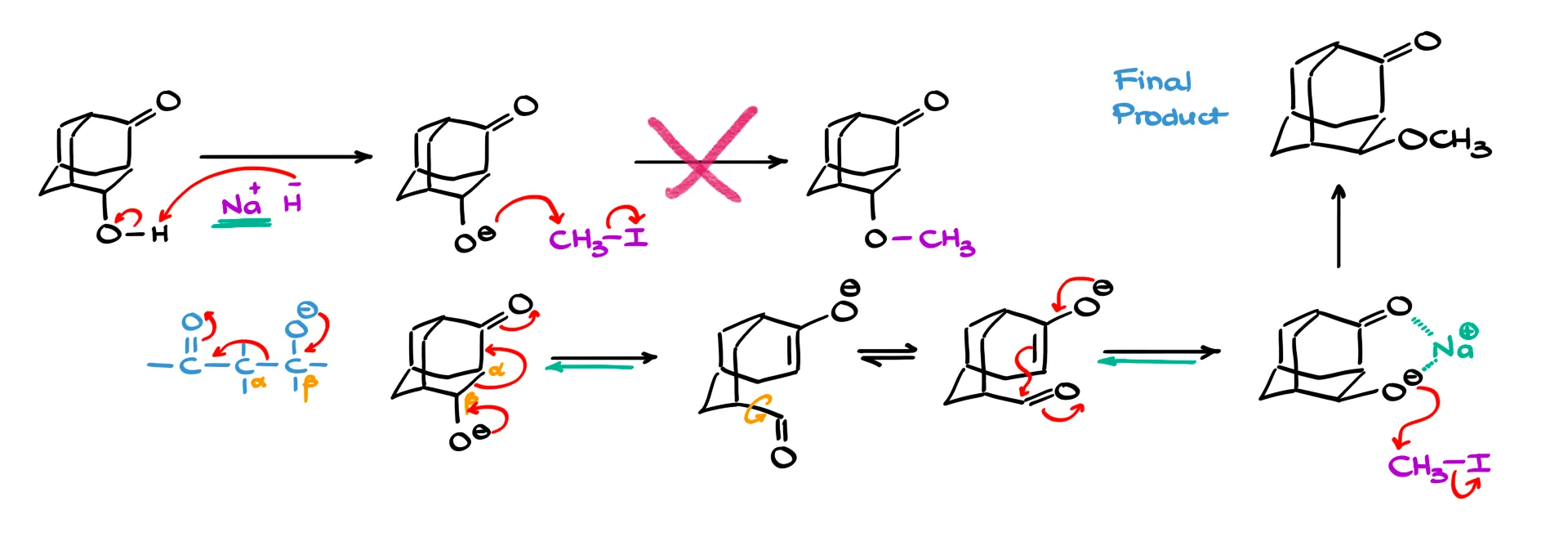
Now, if we just added our alkyl halide and ran a straightforward SN2 reaction, we’d get the expected ether. But since we know that doesn’t happen, something else must be going on.
Let’s look more closely at the alkoxide intermediate. The negatively charged oxygen is sitting on the β-carbon, and this type of species resembles an intermediate in aldol addition or condensation reactions. And since those reactions are equilibrium processes, it means we can actually go backward and break the bond between the α- and β-carbons through a cascade of electron movement.
So here’s what happens: the lone pair on the oxygen moves down to form a carbon–oxygen double bond, while the bond between the α- and β-carbons breaks. This gives us an enolate intermediate.
Now, here’s the cool part: the enolate can freely rotate around the single bond next to the carbonyl. That means the portion of the molecule containing the new aldehyde can rotate, and after rotation, the enolate can re-react with the carbonyl, reforming the α,β bond. The difference is that now the oxygen will be pointing in the opposite direction, effectively inverting the stereochemistry.
The enolate and aldehyde can reform the original α,β bond, but now with the oxygen flipped to the other side.
Why is this new conformation more stable? There are a few reasons, but the key factor is the sodium ion from our sodium hydride reagent. That sodium cation doesn’t just disappear, it stays around as a counterion and coordinates simultaneously to both oxygen atoms. This chelation stabilizes the conformation and holds the molecule in this new, more favorable geometry.
So when we move on to the next step and introduce methyl iodide, this stabilized conformation predominates in solution. The methyl iodide reacts with this form of the molecule, giving us the final ether product, but now with the opposite stereochemical configuration.
What looked like a simple inversion of configuration wasn’t actually an SN2 process at all. Instead, the molecule temporarily broke apart and then reassembled in a new arrangement.
How did you do? Were you able to figure out this mechanism before seeing the explanation? Let me know in the comments below.
