Hydrogen Bonding
In this tutorial I want to talk about hydrogen bonding. Hydrogen bonding is one of the most important intermolecular forces in organic chemistry, yet it’s often glossed over in class with just a quick mention of water or ammonia. In this tutorial, I’ll break down what hydrogen bonding really is, why it matters, which functional groups can participate, and how to avoid the common mistakes students make when drawing or predicting it.
What is Hydrogen Bonding?
So first of all, what exactly is hydrogen bonding? In general chemistry you probably just saw an example with water or maybe ammonia, and that was about it. Now in organic chemistry, most instructors assume you already know what hydrogen bonding is, so they don’t really spend much time on it. That leaves us in this weird situation where nobody really explains it, and everyone assumes it will either be covered later or that you’ve already learned it somewhere else.
Let’s remedy this unfortunate situation!
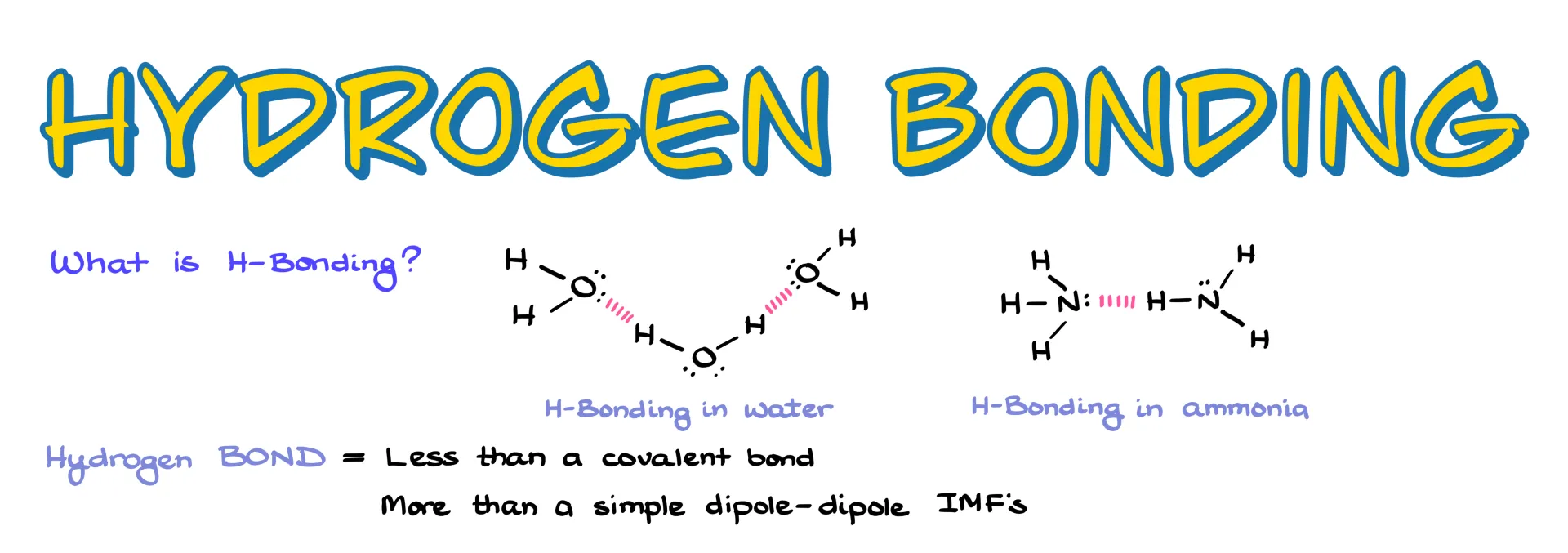
I want to start by pointing out that the term “hydrogen bond” is a bit of a misnomer. It isn’t a real chemical bond in the sense of being covalent, but it’s also more than just a simple dipole–dipole interaction. That’s why we put it into its own category.
By definition, hydrogen bonding is an interaction involving three atoms where hydrogen sits in the middle, sandwiched between two heteroatoms. Typically those atoms are nitrogen, oxygen, or fluorine. While other elements can sometimes participate, it’s usually only to a very small extent, so we focus on these three.
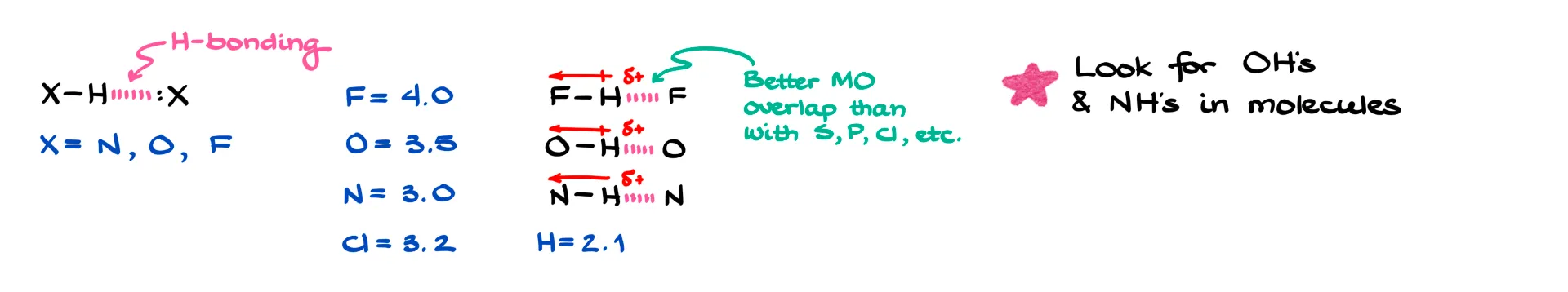
There are two main reasons for that. First, N, O, and F are the most electronegative elements. Because they pull electron density so strongly, the hydrogen attached to them becomes highly polarized and carries a significant δ+. That strong partial positive charge on hydrogen is essential. Without it, hydrogen bonding won’t happen. Second, these are second-period elements, which means their orbitals overlap well with the tiny hydrogen orbital. Larger atoms like sulfur, phosphorus, or even chlorine just don’t overlap as effectively, so they aren’t nearly as good for hydrogen bonding.
So when it comes to organic molecules, the classic cases we focus on are O–H and N–H bonds. Hydrogen fluoride is also a strong hydrogen-bonding molecule, but HF exists as its own compound rather than part of larger organic molecules, so we don’t usually talk about it in this context.
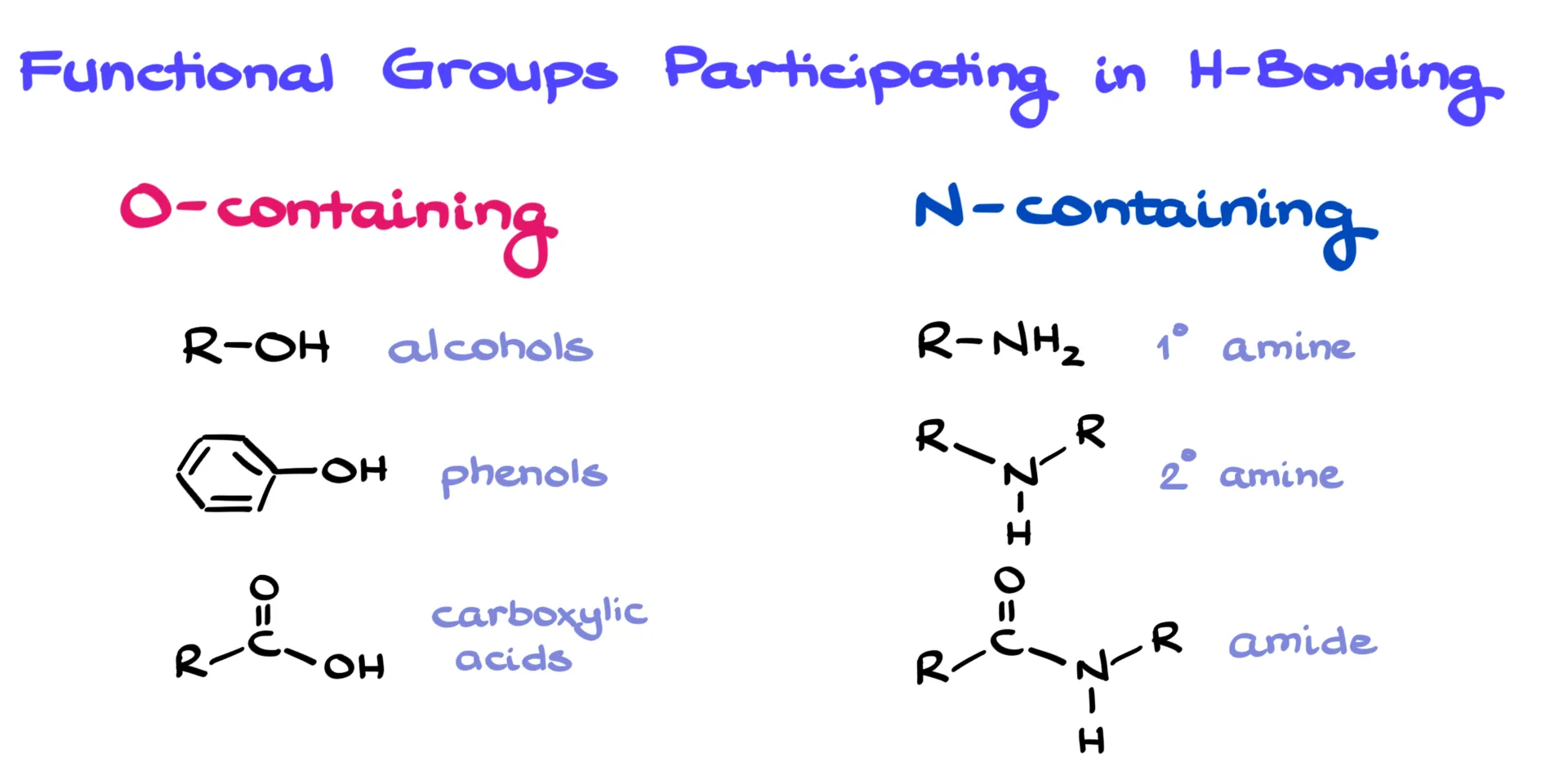
Functional Groups That Participate in Hydrogen Bonding
Since O–H and N–H bonds are what we look for, it helps to review which functional groups contain them. On the oxygen side, we have alcohols, phenols, and carboxylic acids. On the nitrogen side, we mainly see primary and secondary amines and amides. All of these can both donate and accept hydrogen bonds.
The group with the hydrogen is the hydrogen-bond donor, and the group with the electron pair is the acceptor. The same functional group can often be both. But here’s the tricky part: there are plenty of functional groups that contain oxygen or nitrogen but don’t have any hydrogens attached. Ketones, esters, and tertiary amides are classic examples. These groups can’t be donors because they lack N–H or O–H bonds, but they can still act as acceptors thanks to their lone pairs.
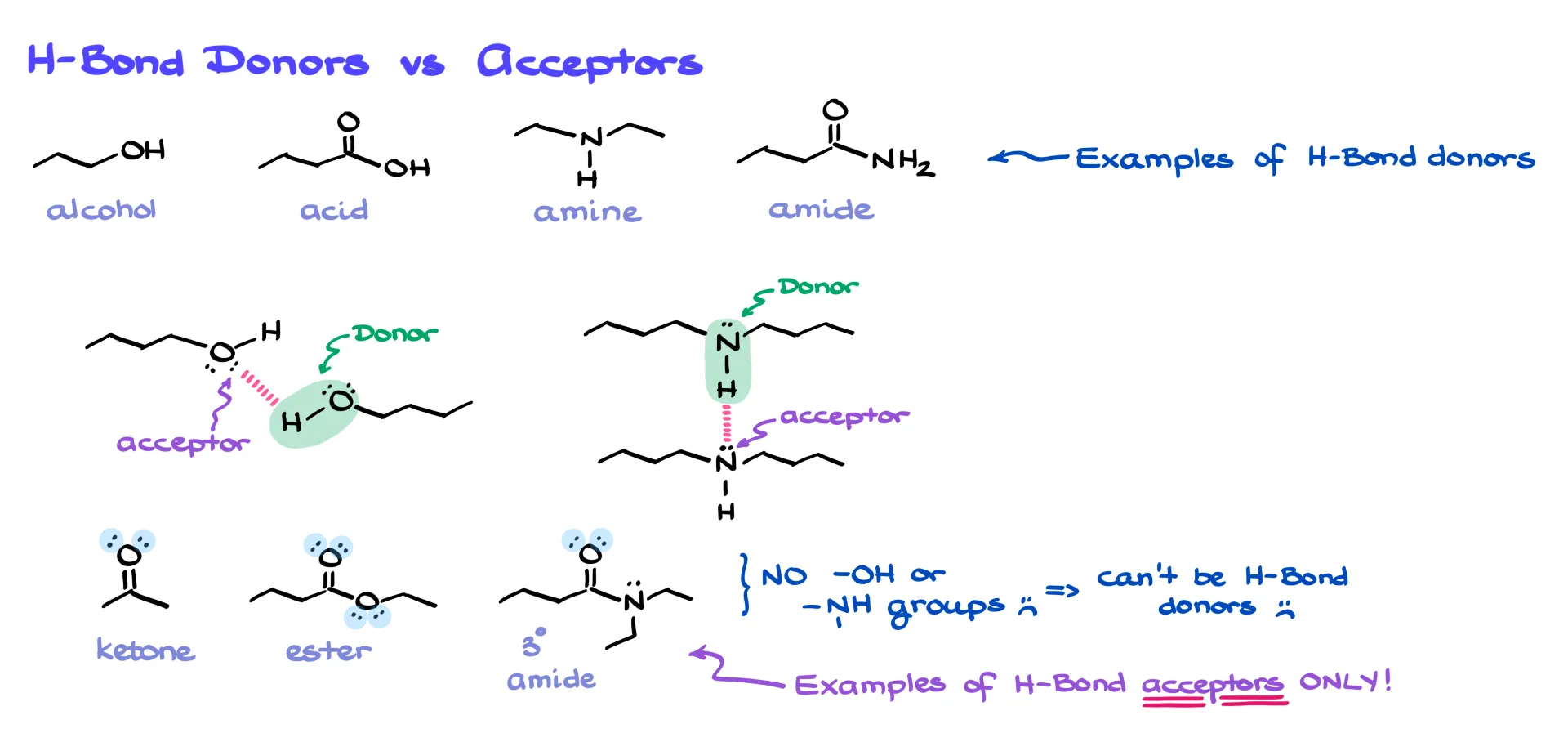
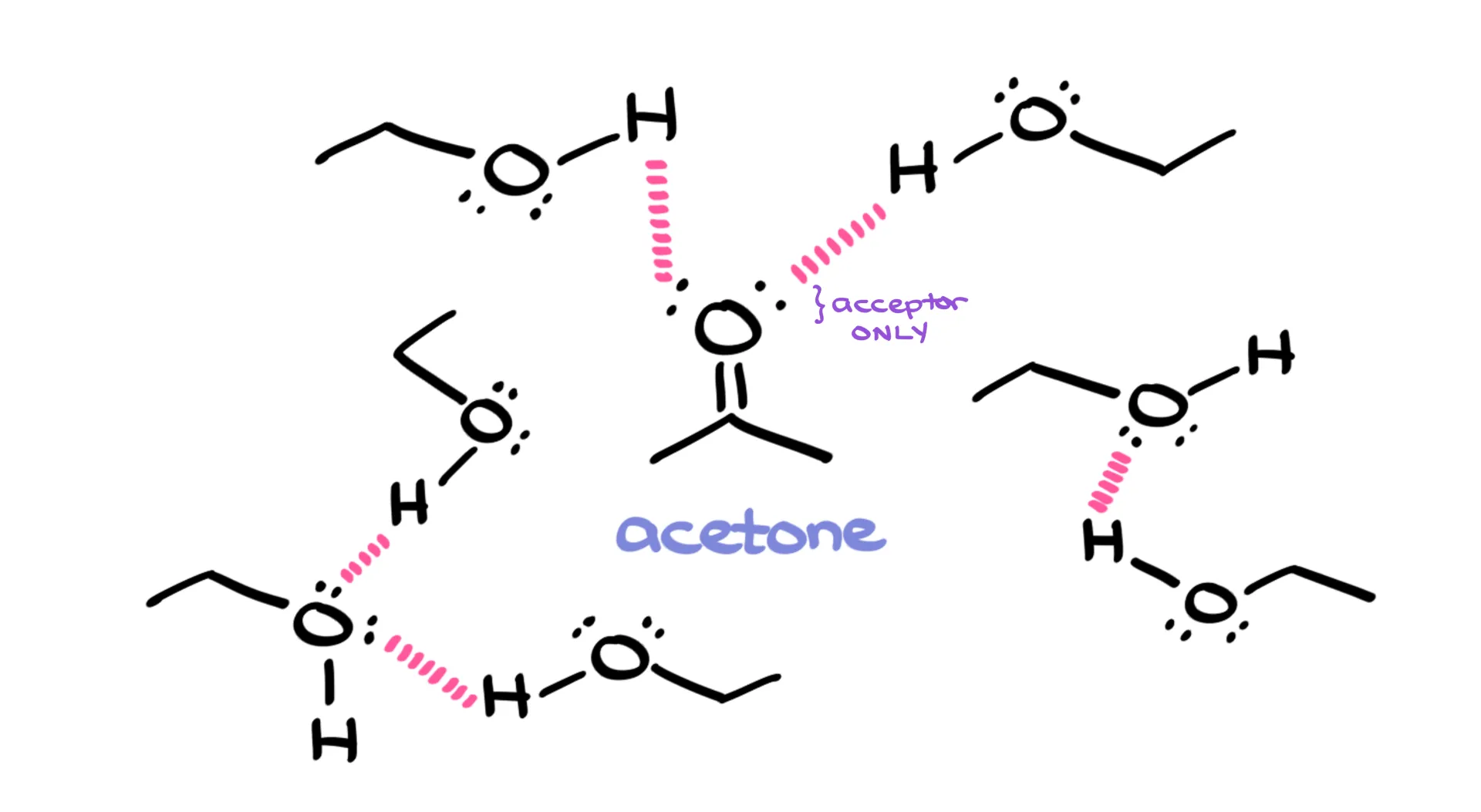
Take acetone, for instance. On its own, acetone doesn’t hydrogen-bond because it can only accept. But if you dissolve it in ethanol, suddenly it participates. Ethanol can donate and accept, while acetone accepts. Together, they form hydrogen bonds. So remember: just because a molecule can’t be both a donor and an acceptor doesn’t mean it can’t participate at all. Context matters.
Examples
Let’s think through some examples. If I show you an alcohol, it has an O–H, so it can both donate and accept. If I show you a lactam, or cyclic amide, it doesn’t have O–H or N–H, but it does have N and O atoms, so it can accept. A molecule with both a ketone and an alcohol can donate and accept, while an alkyl halide—despite chlorine being electronegative—won’t participate because we don’t count C–Cl bonds for hydrogen bonding.

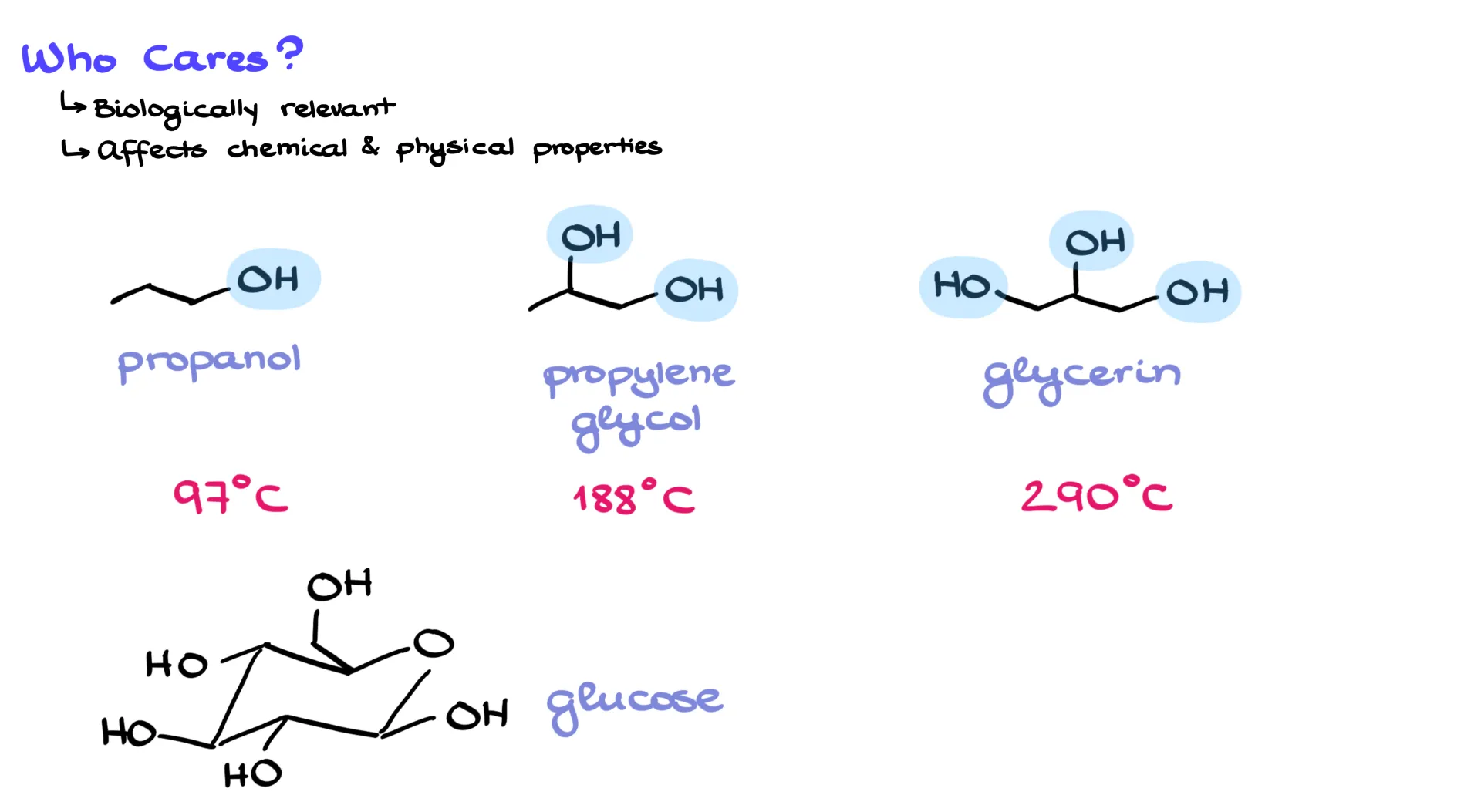
So why do we care? Well, hydrogen bonding plays a huge role in both chemical and physical properties. Without it, life as we know it wouldn’t exist. For example, compare propanol, propylene glycol, and glycerol. These molecules differ only in how many O–H groups they have, but their boiling points are drastically different: 97 °C for propanol, 188 °C for propylene glycol, and 290 °C for glycerol. Each extra O–H raises the boiling point by about 90 degrees. Add even more O–H groups, as in glucose, and the compound is a solid. Try heating it, and instead of boiling it just decomposes into caramel. The hydrogen bonds are so strong that the molecules literally break apart before they can separate from one another.
Hydrogen bonding is also essential in biology. Protein folding, DNA base pairing, enzyme activity—these all depend on it. I won’t go into those details here, but if you take biochemistry or molecular biology, you’ll revisit this many times.
Common Mistakes
Now, let’s talk about some common mistakes.
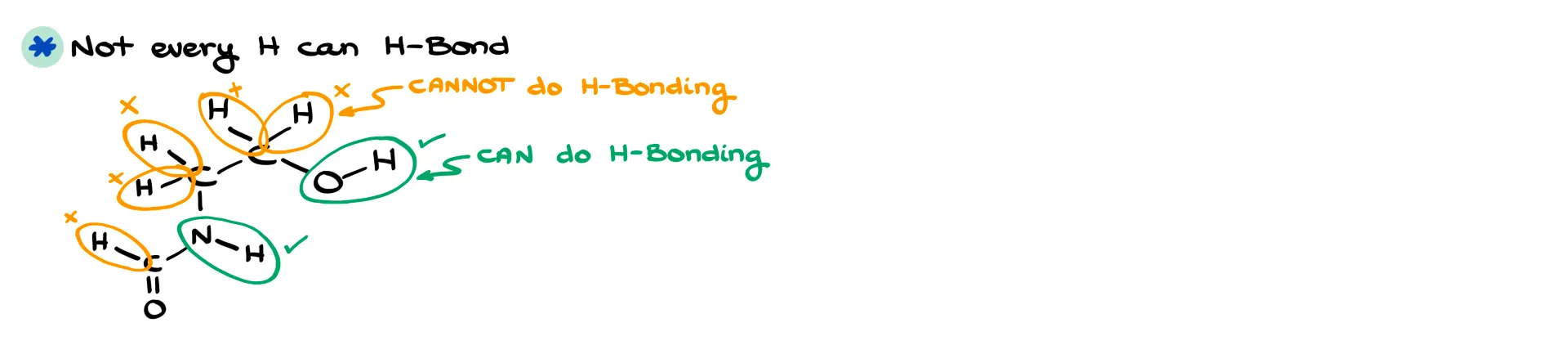
The first is slapping hydrogen bonding onto any hydrogen atom. I can’t tell you how many times I’ve seen students try to draw hydrogen bonding in something like methane. Just because a molecule has hydrogens doesn’t mean they participate. Hydrogens attached to carbon don’t carry the strong δ+ charge we need, so they don’t hydrogen-bond. Only hydrogens attached to highly electronegative atoms like N, O, or F can participate.
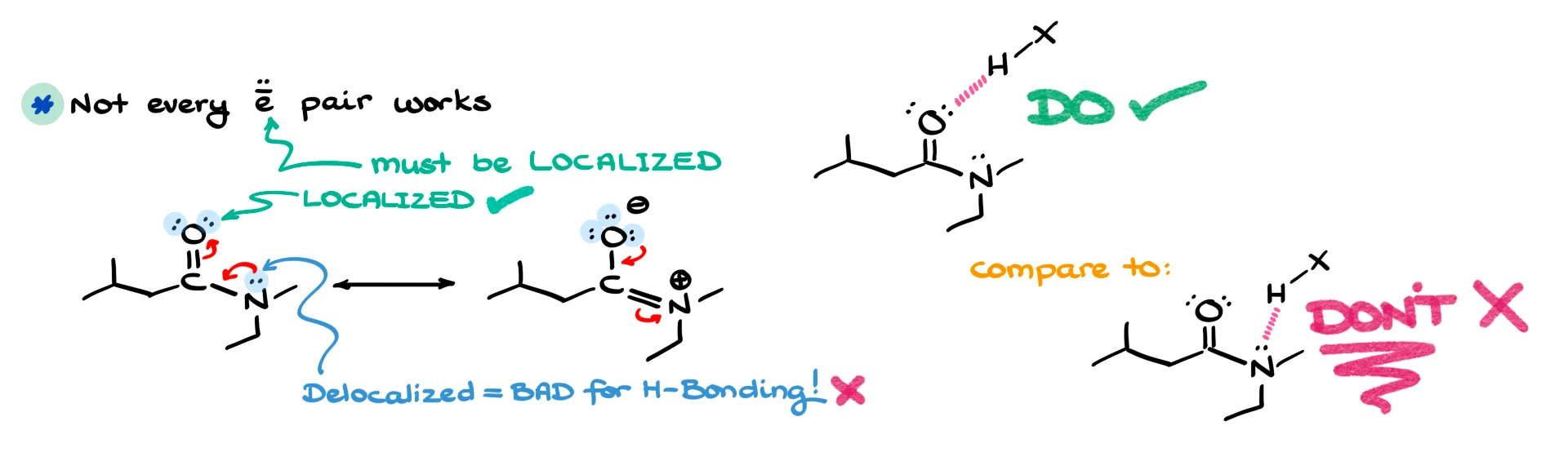
The second mistake is assuming any lone pair can act in hydrogen bonding. Not all electron pairs are equal. Only localized lone pairs participate effectively. If a lone pair is delocalized through resonance, it’s much less available. For example, in an amide, both oxygen and nitrogen have lone pairs, but the nitrogen’s are delocalized. That means hydrogen bonding will primarily occur through the oxygen. Instructors love to use this as a trick question, so be careful.
So, what do you think about hydrogen bonding? Does it still feel like a boogeyman to avoid, or do you feel more confident about tackling it on the test?
