Hydrogenation (Reduction) of Alkenes
Sometimes, we just need to erase the double bond—and if that’s what you’re trying to do to get your target molecule, hydrogenation is the reaction you need. In this tutorial, we’ll go over the ins and outs of this simple yet powerful reaction that converts alkenes into alkanes.
Mechanism of the Alkene Hydrogenation
Now, I’d usually start with a mechanism like I do for most reactions, but in this case, we don’t actually know the exact mechanism. And if anyone tells you otherwise, they’re not being honest. Don’t get me wrong—we have a pretty good idea of what’s going on, but there isn’t really a mechanism in the traditional sense of curved arrows and step-by-step electron movements.
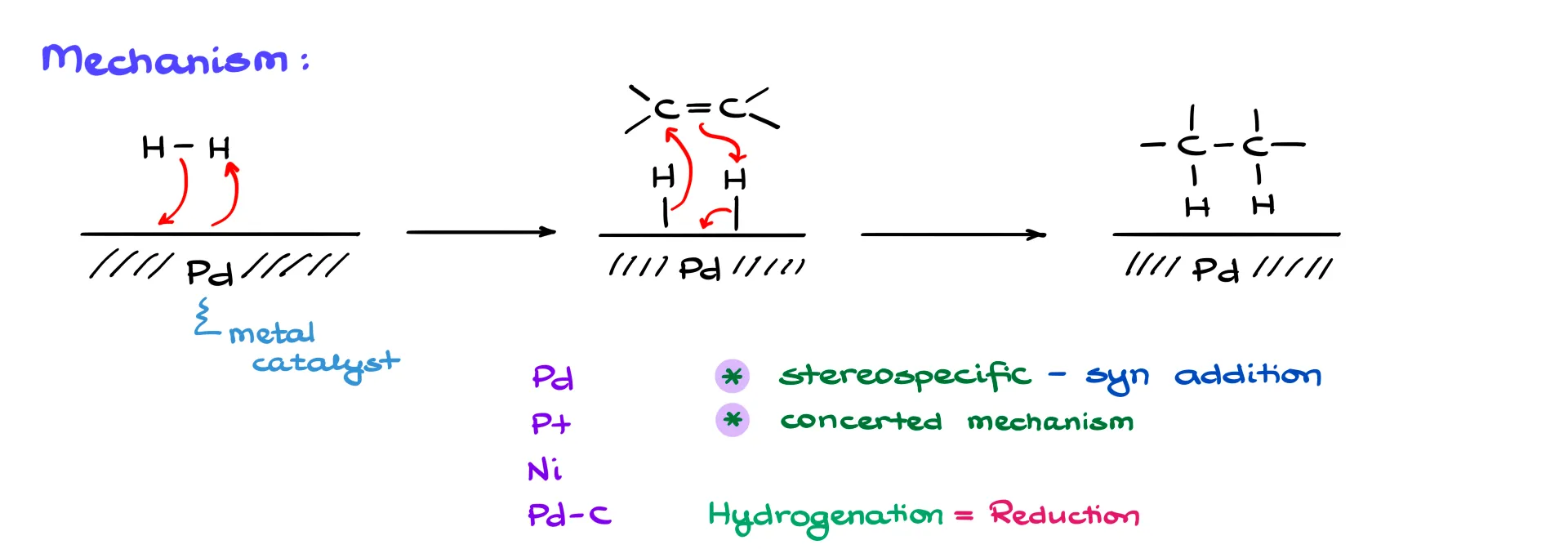
Here’s what we do know: the mechanism starts with the absorption of H₂ onto a metal catalyst. That catalyst can be palladium, platinum, nickel, or even palladium on carbon—what I like to call the poor chemist’s catalyst, since it’s much cheaper than pure palladium powder.
Once we’ve got the catalyst, it absorbs the hydrogen. If you really want curved arrows, you could imagine the electrons going from H₂ to the palladium and then from the palladium to another hydrogen. Basically, we end up with atomic hydrogen stuck to the surface of the metal.
When the double bond comes in, and it picks up those hydrogens. Again, if you’re desperate for arrows, you can say electrons go to one carbon, the π-bond donates to a hydrogen, and those electrons go onto the palladium. Something along those lines. As a result, we end up with an alkane, now bearing those two hydrogens, and the palladium is free to go again for the next cycle.
This reaction is stereospecific—specifically, it’s a syn addition. That means both hydrogens are added to the same side of the molecule. Is it 100% stereospecific? Not quite. In some cases, you might get up to 20% of the anti-product, but for the scope of a typical sophomore organic course, we treat it as strictly stereospecific. So yes, for our purposes, it’s syn all the way.
We also postulate that this reaction is concerted—both hydrogens add at the same time. That’s not entirely confirmed by experimental data, but again, within our course, we treat it as a concerted mechanism.
And just so you know: the term “hydrogenation” is interchangeable with “reduction” in this context. So you might hear it referred to as either the hydrogenation of an alkene or the reduction of an alkene—both are perfectly valid and mean the same thing.
Hydrogenation of Alkenes Examples
Now, let’s get into an example. I’ll start with 1,2-dimethylcyclohexene—my new best friend. Why better than methylcyclohexene? Because it has two methyl groups, obviously.
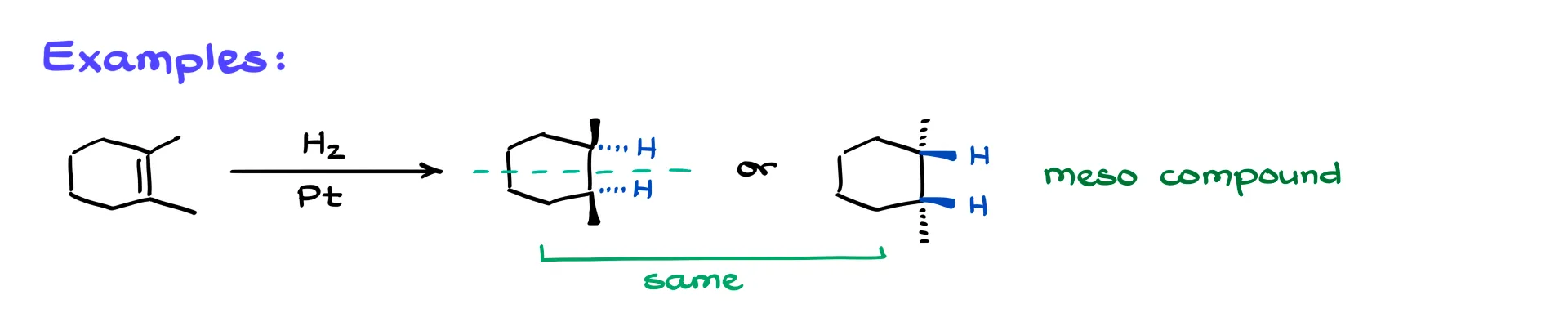
In this case, we add two hydrogens to the molecule. Let’s highlight them in blue. If I show the attack coming from the back face, then the methyl groups get pushed to face forward, so they’ll both be on wedges in the final structure. Alternatively, if the hydrogens come from the front face, we get the mirror image.
Now, what’s the relationship between these two molecules? If you said “enantiomers,” I caught you—they’re actually the same compound. It’s a meso compound because it has an internal plane of symmetry. So once again, stereochemistry matters a lot.
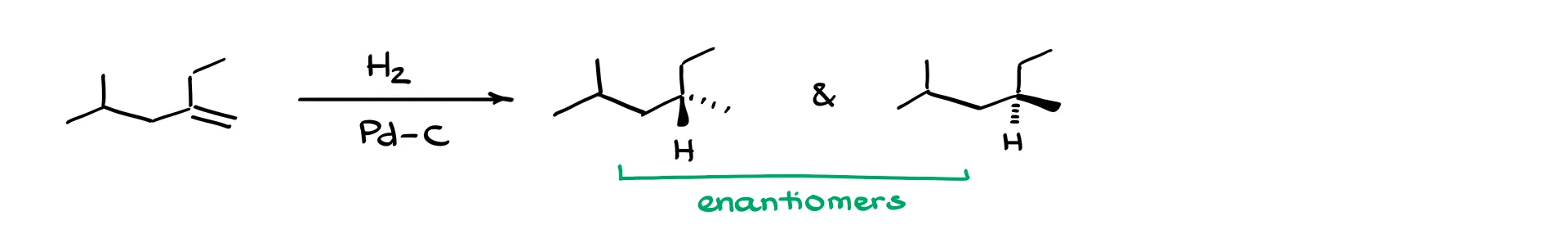
Let’s look at another example. We’ve got a double bond again and we’re adding hydrogens from the front face. That gives us a product with two hydrogens both facing forward. Alternatively, they could add from the back face. Now, the carbon atoms on the right side aren’t chiral, so showing stereochemistry there is unnecessary—I’ll just erase those hydrogens and make them implicit.
But on the left side, those carbons are chiral. To make this clearer, I’ll show one bond as a dash and the other as a wedge. These two molecules are now true enantiomers because each has a chiral center that is the mirror image of the other.
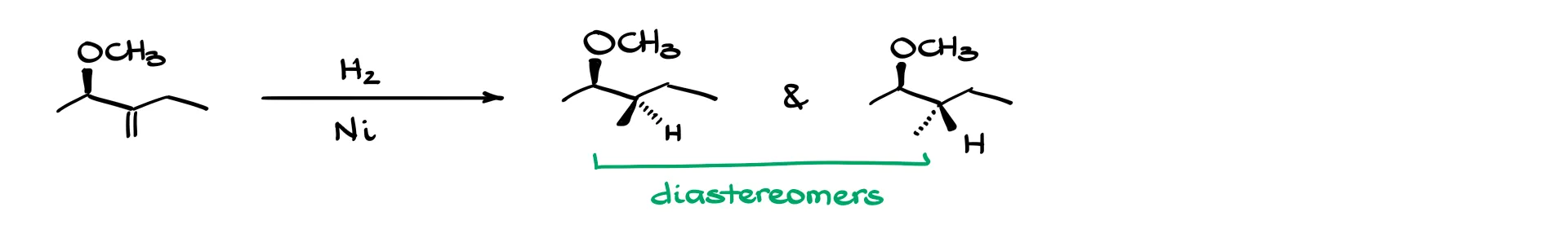
Here’s one more good case: I add hydrogens across a double bond, either both from the back side or both from the front. The CH₃ group at the bottom isn’t chiral, so I’ll remove the stereochemistry from that part. Instead, I’ll focus on the methyl group that gets pushed around by the hydrogen—it’s on a wedge in one product and a dash in the other.
In this scenario, the two products are diastereomers. That’s because we already had a chiral center in the molecule—at the methoxy group—and that center stays the same. Since only one of the chiral centers changed, the two molecules are not mirror images or superimposable. Diastereomers!
So, hydrogenation is simple and straightforward—it gets rid of the double bond and adds hydrogens in a syn fashion. We won’t ask you to show the mechanism, which is a nice bonus. To draw the product, just take the double bond out and stick on two hydrogens—either on wedges or dashes, depending on your face of attack.
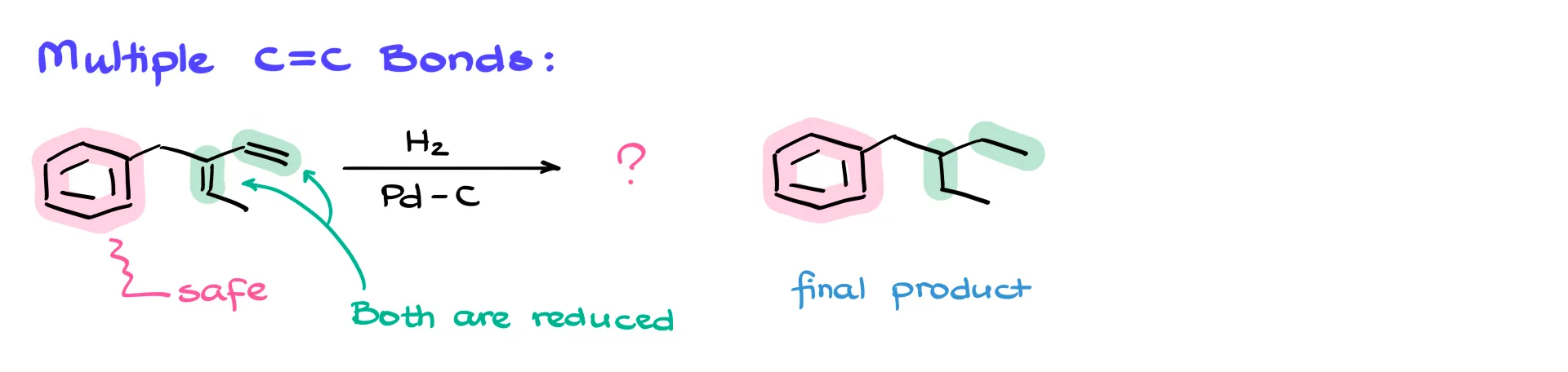
One important note: if your molecule has multiple double bonds, like in this next example, all of them will be reduced. The only exception is the aromatic ring—it stays safe unless you’re working with extremely high pressures and temperatures. So here, the final product will have both double bonds reduced, but the aromatic ring remains untouched.
And that’s about it—no tricks, no weird exceptions. This reaction really is as simple as it looks.

Hi Victor!
Usually at which pressure hydrogenation reaction proceeds or pressure isn’t necessary?
In most cases just a couple of atmospheres of pressure is sufficient. Usually, I see 2-5atm in most typical procedures.