Carboxylic Acids and Carboxylic Acid Derivatives
Carboxylic acid functional group is very common in organic synthesis and in biochemical processes. Thus, knowing the reactions of carboxylic acids is a must for anyone who wants to master organic chemistry.
Acid-Base Properties of Carboxylic Acids
Carboxylic acids are, well, acids 😀 so they tend to dissociate giving a proton/hydronium ion and a corresponding conjugate base. Carboxylic acids are considered relatively weak acids with typical pKa values between 4 and 6. For instance, acetic acid (the one that you find in table vinegar) has pKa=4.75 while a sulfuric acid (your typical strong acid) has pKa=-3. So, your typical strong acid is over ten million times more acidic than a typical carboxylic acid.
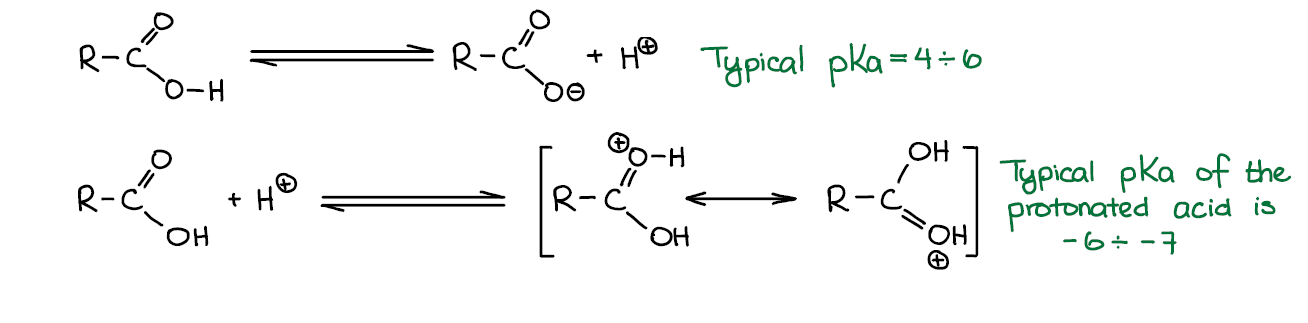
When in present of a strong acid, carboxylic acids can be protonated. However, protonated carboxylic acids are unstable and are extremely acidic and electrophilic with typical pKa’s around -6 to -7. While it is essential to protonate carboxylic acid for some carboxylic acids reactions such as Fischer esterification, they require a strong acid to do so and the protonated version will never be present in a solution in a high concentration.
Fischer Esterification
This esterification reaction is named after Emil Fischer who received a Nobel Prize in chemistry “in recognition of the extraordinary services he has rendered by his work on sugar and purine syntheses.” He’s the person who investigated the reaction the most and is considered esterification discoverer.
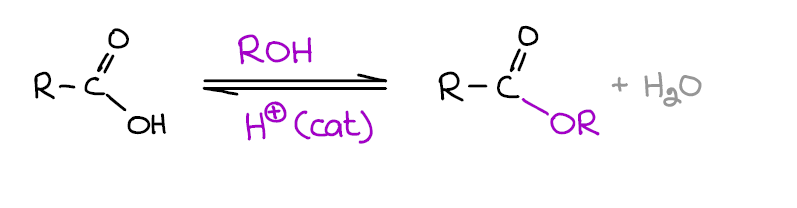
This reaction is one of those must-know reactions of carboxylic acids that you are definitely going to see on your exams, quizzes, homework, and MCAT. Two important point you wanna remember about this reaction:
- It’s an equilibrium! This means that Fischer esterification can easily go in the forward or reverse direction according to Le Chatelier’s principle. So if you want to force the formation of an ester, add more acid or alcohol, or remove one of the products. The most typical method of forcing this equilibrium towards the ester is removing of water with the Dean-Stark trap. If you want to hydrolyze your ester instead, just add more water!
- This reaction requires a strong acid catalyst. So it might not be the best option when either the acid or the alcohol are sensitive to acids. Notably, tertiary alcohols tend to dehydrate giving alkenes in these conditions.
Fischer esterification can be used to make lactones (cyclic esters). Lactones are important derivatives of carboxylic acids in both organic synthesis and in some biochemical processes.
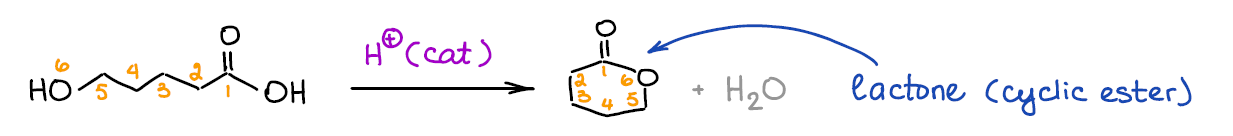
Generally, Fischer esterification will favor 5- and 6-membered lactones. While other ring sizes are theoretically possible, we rarely see those actually formed. Still, you should keep an eye on those as they may show up on the test.
Conversion of Carboxylic Acids into Acid Halides
This is one of those important reactions of carboxylic acids that you’re going to find yourself doing over and over again in your homework and on the test. Carboxylic acids themselves are not particularly electrophilic and shy away from most nucleophiles. Acid chlorides, on the other hand, are very aggressive electrophiles and will readily react with what we consider as weak nucleophiles like water or alcohols.
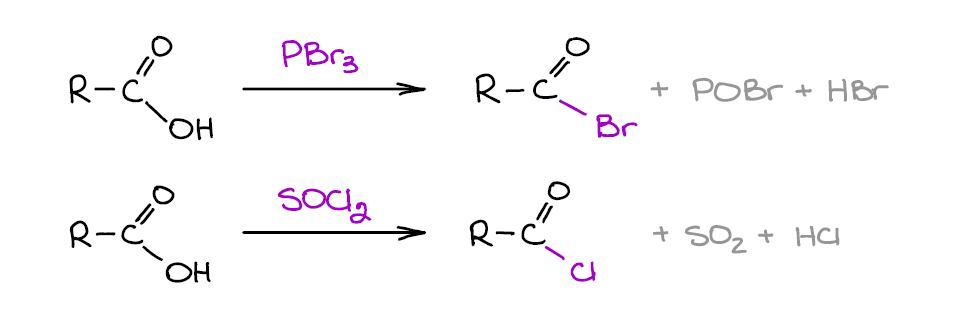
You’ll mostly be dealing with acid chlorides, so SOCl2 will be your usual go-to reagent. Bromides, however, are also somewhat common and you may see your instructor throwing those in the mix on the test. Acid chlorides are also more reactive towards the nucleophiles.
Formation of Acid Anhydrides
Anhydrides are similar in their reactivity to acid halides. They are less useful for the synthesis and you won’t see them as a focus of many questions. Anhydrides can be simple and mixed.
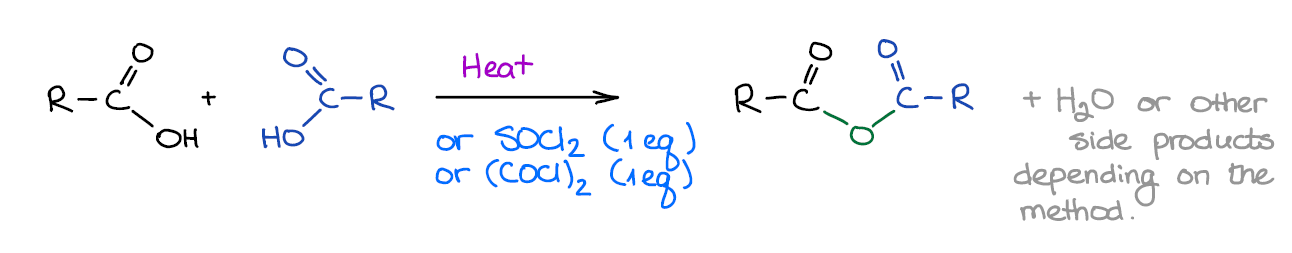
Acid anhydrides don’t play much of a role in pretty much any relevant chemistry, so you just gotta remember that they are similar to chlorides reactivity-wise, give a carboxylic acids as side products with nucleophiles, and that’s about it.
Reduction of Carboxylic Acids with Lithium Aluminium Hydride (LAH)
LAH is a very useful and a very common reducing agent in organic chemistry. It is very strong and will easily reduce carboxylic acids to the corresponding primary alcohols.
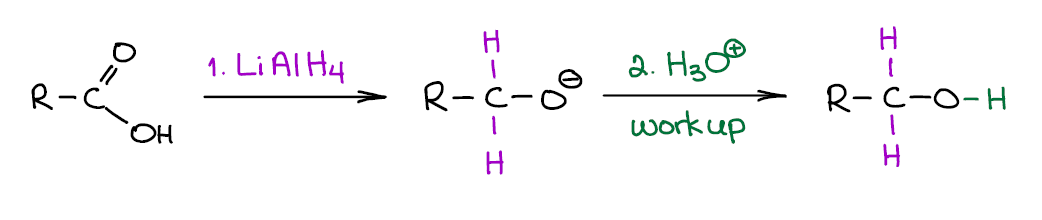
The first step of the reaction is very exothermic and produces molecular hydrogen (H2) and may end up in an explosion if not performed carefully. It also produces a corresponding alcoholate (a deprotonated alcohol) as a product, so you’ll need to use the acidic workup to get the neutral alcohol. Note though, some instructors have tendency to “skip” or “assume” the workup steps, so be very careful with how your instructor writes their reactions. If they routinely assume the workup step, you’re safe writing the alcohol product right the way. If, however, they show the workup step and you forget about it on the test, you’re likely to lose points.
Hell-Volhard-Zelinsky Reaction
Hell-Volhard-Zelinsky or HVZ for short is an important reaction of carboxylic acids. It is actually a two-step sequence rather than just a reaction. The first step makes an α-brominated acid bromide. Then, in the second step you “quench” the bromide from the first step to make either an acid or an ester.
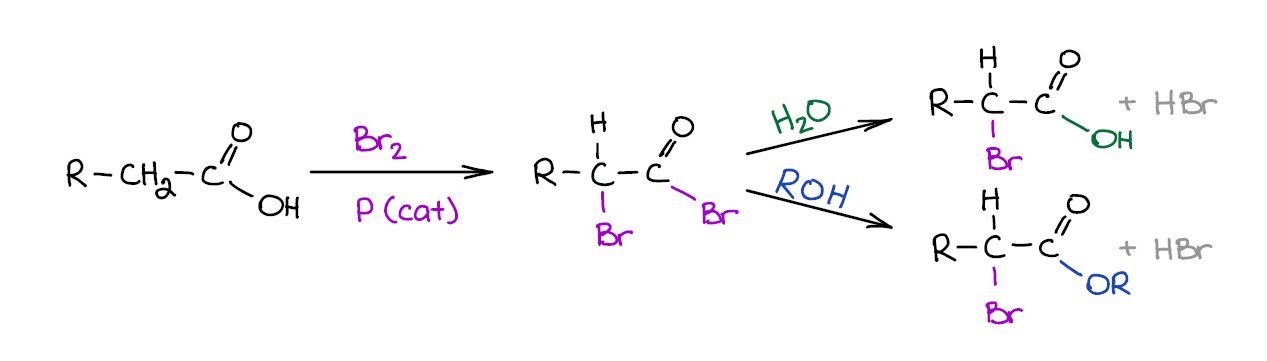
This reaction is often covered in the chapter dealing with enols and enolates as the mechanism involves an acid-promoted enolization. So, if you’re not seeing this reaction among the ones you’re covering in the reactions of carboxylic acids chapter, keep it at the back burner for now as you’ll need it for later.
Decarboxylation of β-Ketocarboxylic Acids
This is another one of those reactions that you’re probably going to see when going over the reactions of enols and enolates. β-Ketocarboxylic acids are unstable and spontaneously decarboxylate at elevated or sometimes even at room temperature.
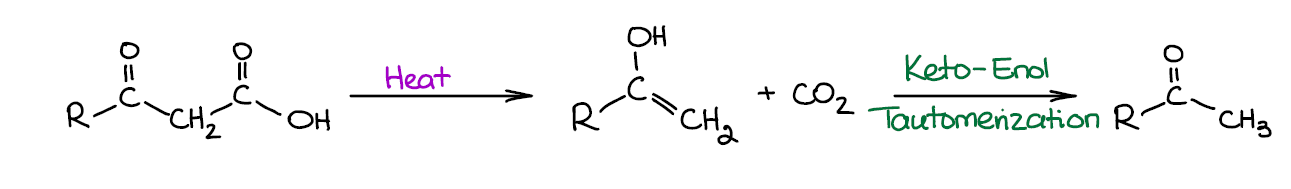
Decarboxylation of β-Ketocarboxylic Acids
This process initially forms an enol. But since enols are significantly less stable than the corresponding ketones, we see a quick keto-enol tautomerization giving the corresponding carbonyl. As we’re working in acidic conditions, the tautomerization is acid catalyzed and proceeds very fast. Basically, you’re not going to have (or even write) the enol form unless you’re doing a complete mechanism for this reaction.
Formation of Amides
Amides are incredibly important for living systems. Peptide bonds, chemically speaking, are amides. So if not for amides, we wouldn’t even exist. Well, maybe we would, but certainly not in the current shape or form. A puddle of ooze would be more like it 😆
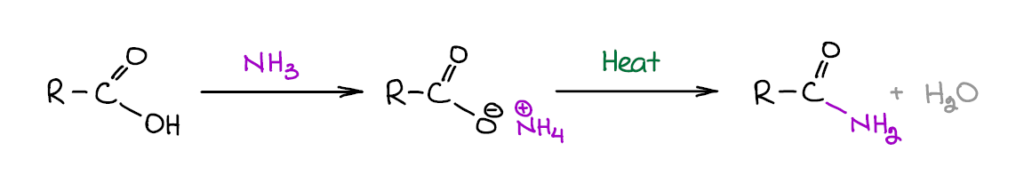
Formation of amides directly from carboxylic acids via dehydration of a corresponding ammonium salt is a very bad idea. While amides themselves and ammonium salts are usually stable at high temperatures, the rest of the molecule might not be. Generally, organic molecules do not tolerate high temperatures well and this reaction requires anywhere between 100°C to 250°C, which may be a bit too extreme for most compounds. So, this reaction is only suitable for making very simple amides. If you’re planning on making something fancy, you’ll probably want to go with some other method like reacting an acid chloride or an ester with an amine.
Activation of Carboxylic Acids with Carbodiimides
Occasionally, activating carboxylic acids by turning them into acid chlorides is not such a good idea. It might be because you have nucleophilic functional groups that will interfere with the reaction, or the molecule itself is sensitive thionyl chloride, or some other reason. In such cases you can use a much more expensive method of converting an -OH of a carboxylic acid into a good leaving group by reacting it with a carbodiimide.
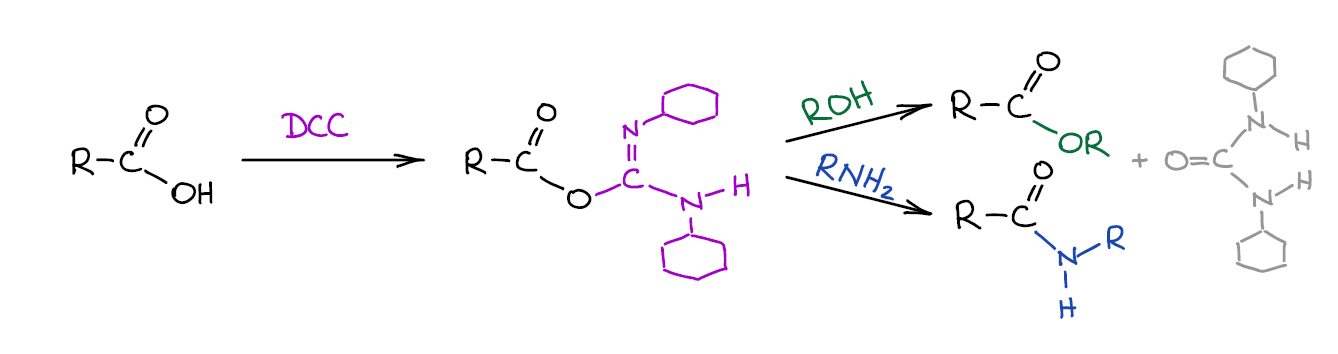
This is a common way of polypeptide synthesis. There are many carbodiimide coupling agents, so it doesn’t have to be DCC. Some instructors skip this synthetic procedure altogether. However, if your course has any biochemistry at the end of the second semester, you’re most likely going to see it then. Carbodiimide coupling is usually covered in a chapter on amino acids and peptides.
Nucleophilic Reactions of Carboxylate Anions
Since carboxylic acids can be easily deprotonated, they form a stable carboxylate ion. Carboxylate ions are negatively charged and only have two resonance structures, so they are somewhat nucleophilic and can undergo SN2 reactions.
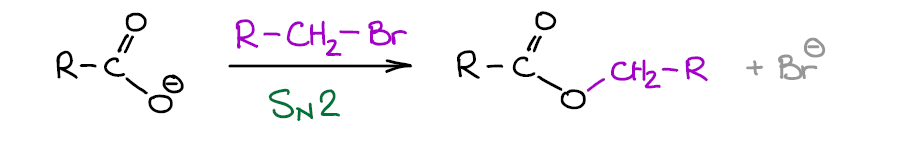
They are weak nucleophile due to the resonance stabilization of the negative charge. They’re not as weak as, for instance, water or alcohols, but it’s not anywhere as good as an alkoxide anion. Basically, this means that carboxylate anions can do SN2, but only if we have an “eager” electrophile such as primary, allylic, and benzylic alkyl halides.
So, that’s your list of must-know reactions of carboxylic acids. There may be a few more that your instructor covers. But those are more of a pet reactions that your instructor might personally like. These reactions, however, are what you’ll need to know for any standardized exam like an ACS or MCAT tests.
If you like to know more about reactions of carboxylic acids, I have reactions of carboxylic acids notes and cheat sheets covering all of these reactions and more. You’ll also get a full description of each reaction with a complete curved-arrow mechanism and notes about important aspects of each step. Reactions of carboxylic acids notes is also included in the complete set of organic chemistry notes and cheat sheets in case you want to pick those up to review for the upcoming test.
