How to Draw Transition States
In this tutorial I want to talk about transition states, and more specifically, how to properly draw them.

Example 1: SN2 Reaction
Let’s start with a familiar example: an SN2 reaction. Here, I have a reaction between methyl iodide and a methoxide anion. Mechanistically, it’s straightforward. The methoxide ion attacks and displaces iodine, which acts as the leaving group.
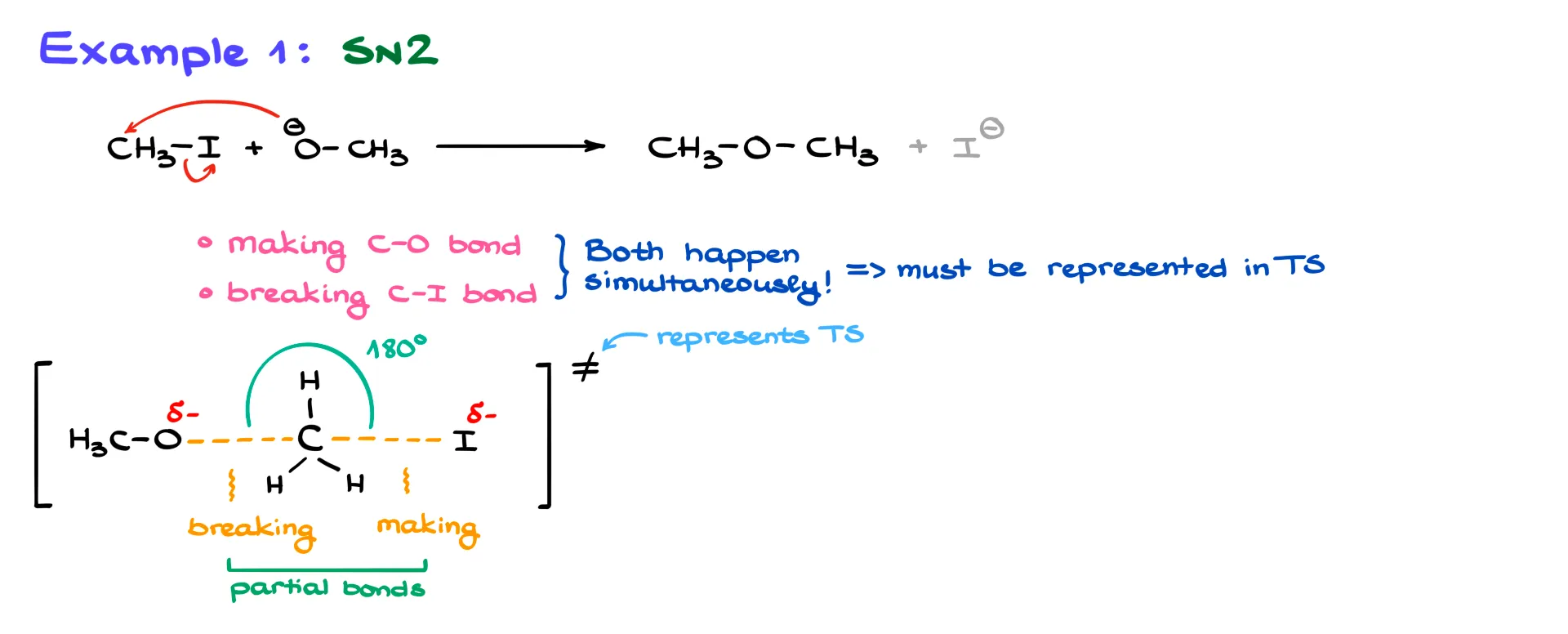
In this process, we are doing two very important things. We are making a new C–O bond, and we are breaking a C–I bond. Both happen simultaneously, which means they must both be represented in our transition state. There are no intermediates here—everything happens at once.
To draw the transition state, I start with the carbon atom. Since I’m making a new carbon–oxygen bond, I show it with a long dashed line. Likewise, I represent the bond between carbon and iodine with a long dashed line because it’s in the process of breaking. And, of course, carbon still has its hydrogens, so I show those too.
Dashed lines are used when representing a bond being broken or made. In other words, they show partial bonds currently in the process of forming or breaking.
Charges are also important. Since oxygen is donating electron density to carbon, I mark oxygen with δ−. Iodine is leaving and gaining electron density, so it gets δ− as well. To show that this is a transition state, I enclose the structure in square brackets and add the crossed equal sign in the corner. This symbol is sometimes called a double dagger, a term that comes from older typesetting where two small dagger symbols were stacked to resemble the crossed equal sign.
Remember to draw proper geometry for your transition states
Geometry matters. In an SN2 reaction, the backside attack means the forming and breaking bonds must be 180° apart. Some instructors may overlook this, but in most cases it’s important and can cost you points. If you draw incorrect bond angles, you’re likely to lose most or all points for that question.

Example 2: SN1 Reaction
Now let’s move to an SN1 reaction. SN1 is a multi-step process, so each step has its own transition state. In this example, we have three steps.
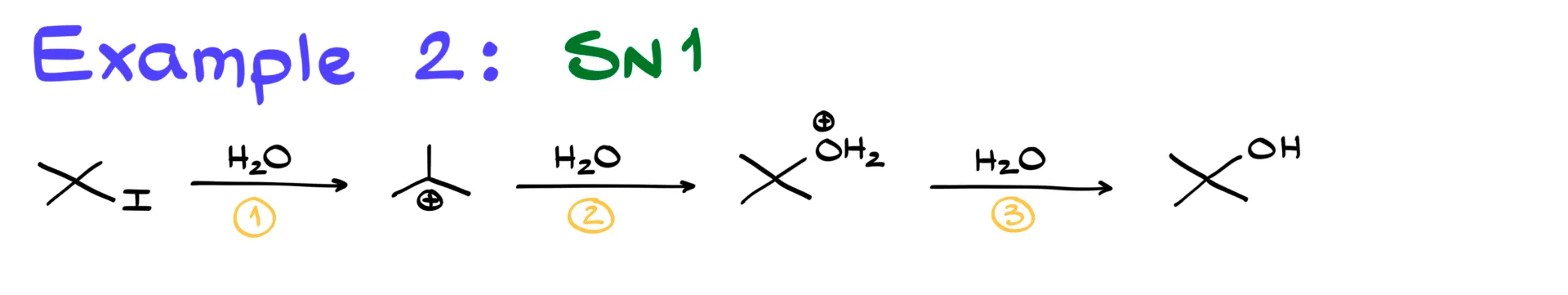
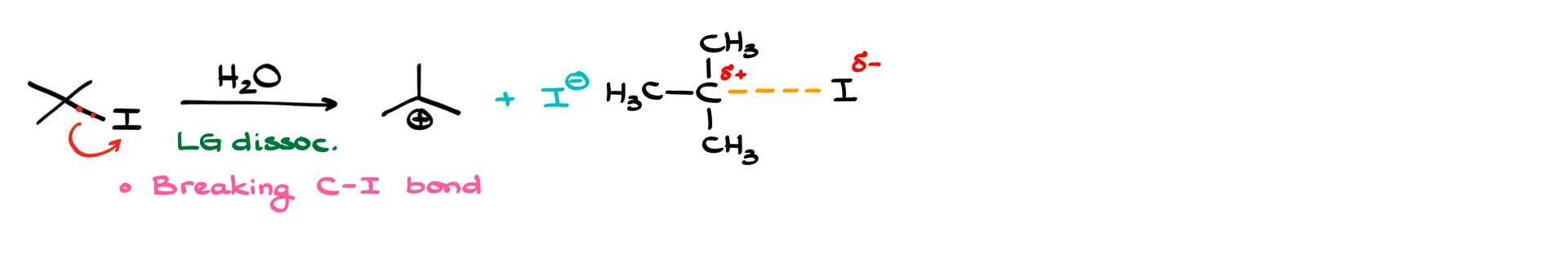
The first step is the loss of the leaving group. We’re only breaking the carbon–iodine bond here, so the transition state shows the carbon with a dashed bond to iodine, a δ− on iodine, and a δ+ on carbon.
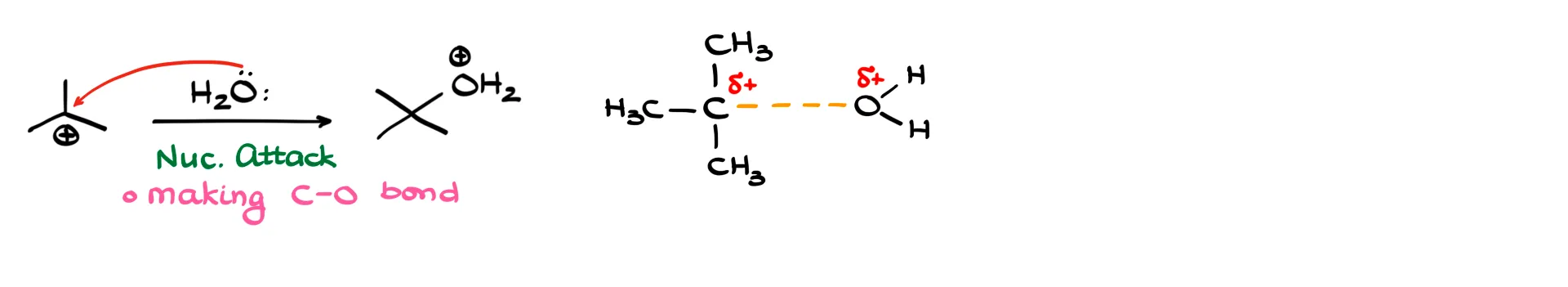
The second step is a nucleophilic attack by water on the carbocation. This forms a new carbon–oxygen bond, shown with a dashed line. Partial charges remain similar, as the positive charge shifts from carbon to oxygen.
The third step is a proton transfer from the organic molecule to water, which acts as a base. Here, one oxygen–hydrogen bond is breaking and another oxygen–hydrogen bond is forming. Both are drawn as long dashed lines, with δ+ on each oxygen. The hydrogen itself does not gain any charge, which is a common mistake to avoid.
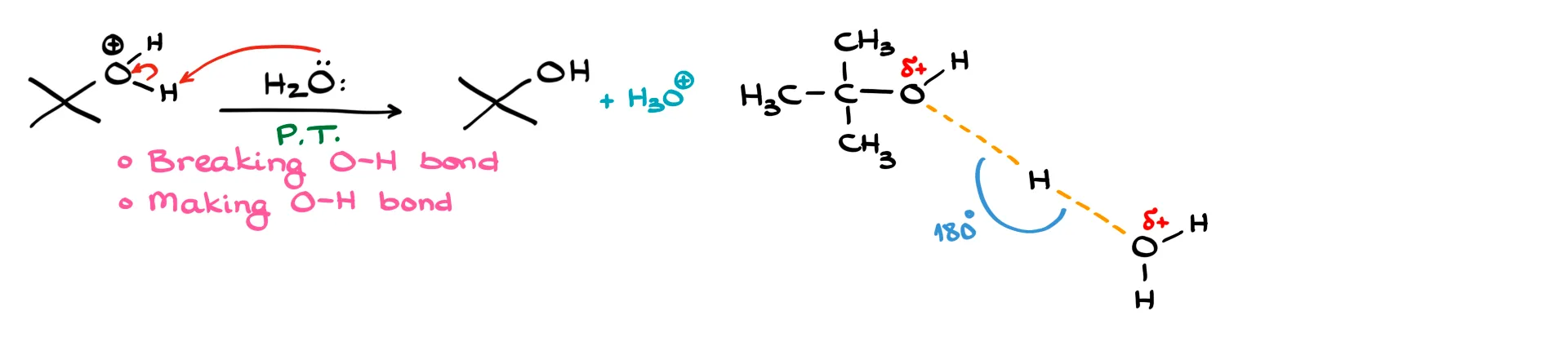
Example 3: E2 Reaction
Next, let’s look at an E2 reaction.

This is also a concerted process like SN2, with everything happening in one step.
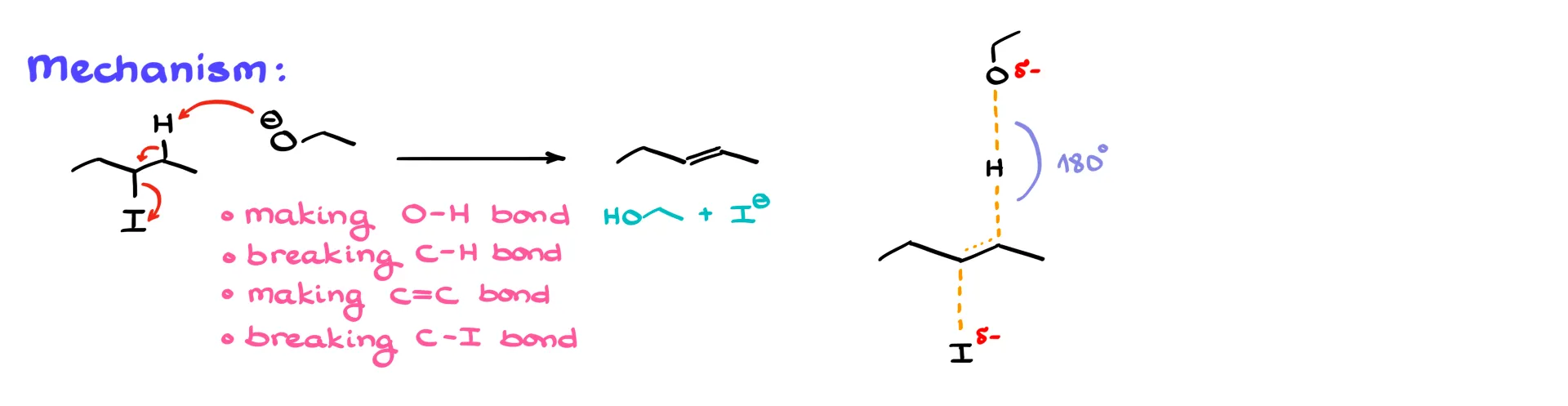
In this example, we are making a new oxygen–hydrogen bond, breaking a carbon–hydrogen bond, making a new carbon–carbon π bond, and breaking a carbon–iodine bond—all in one step. The transition state must show all four bonds being made or broken as dashed lines.
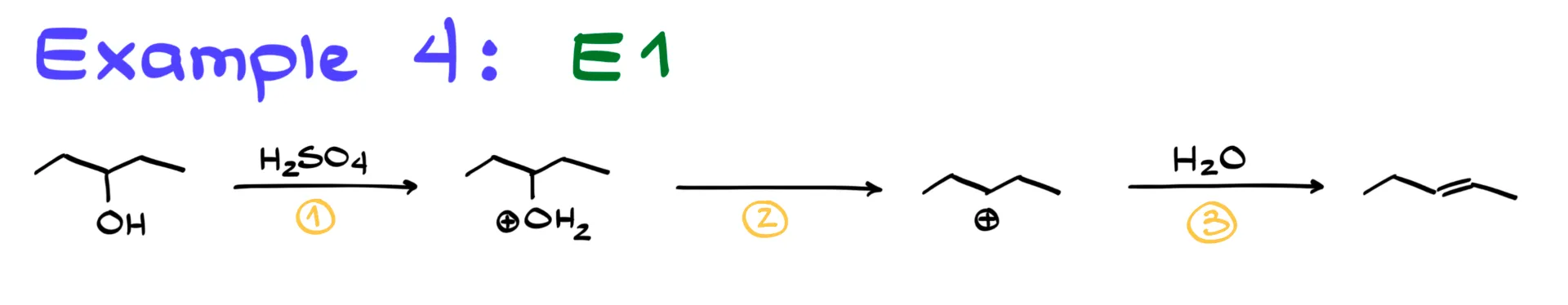
Example 4: E1 Reaction
Finally, let’s look at an E1 reaction. This is a multi-step process similar to SN1.
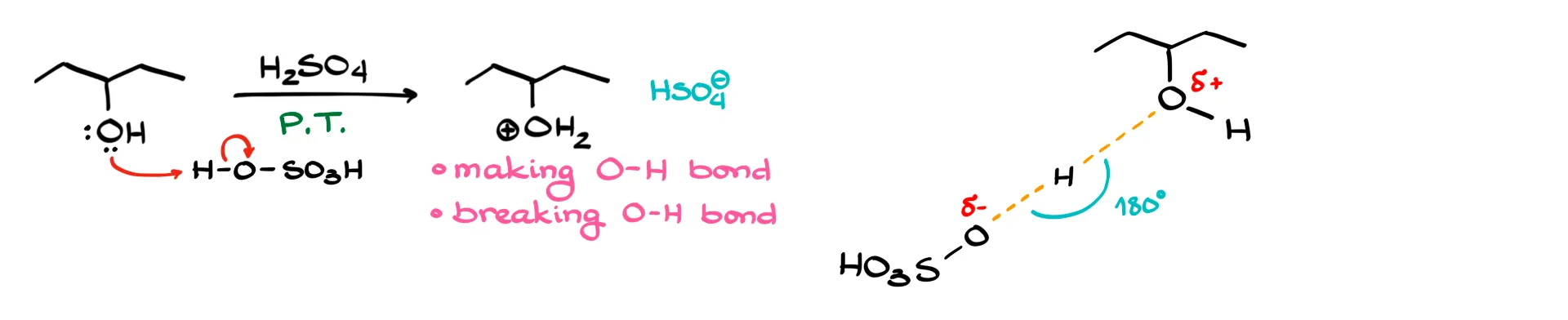
In the first step, we protonate the –OH group. One oxygen–hydrogen bond is formed and another is broken as the proton moves from one oxygen to the other. The transition state keeps bond angles roughly correct, even if the drawing looks awkward.
The second step is the leaving group dissociation, which is straightforward: we break the carbon–oxygen bond. The transition state here is simple, with one dashed line and partial positive charges where appropriate.
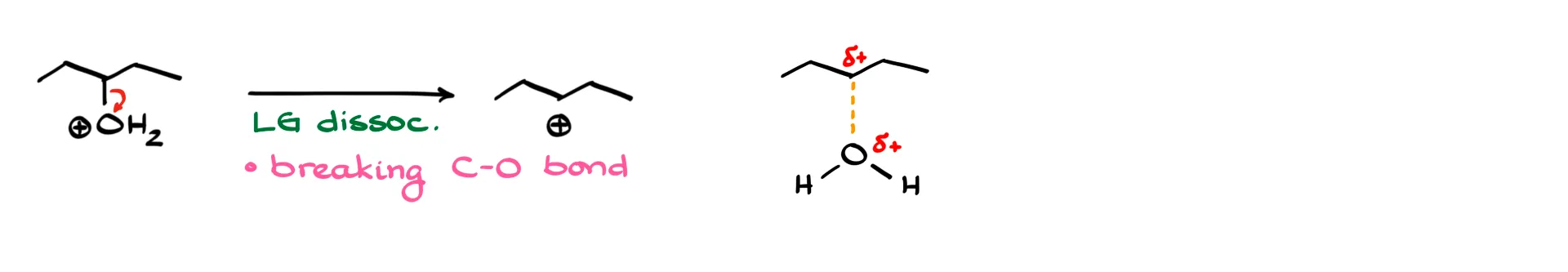
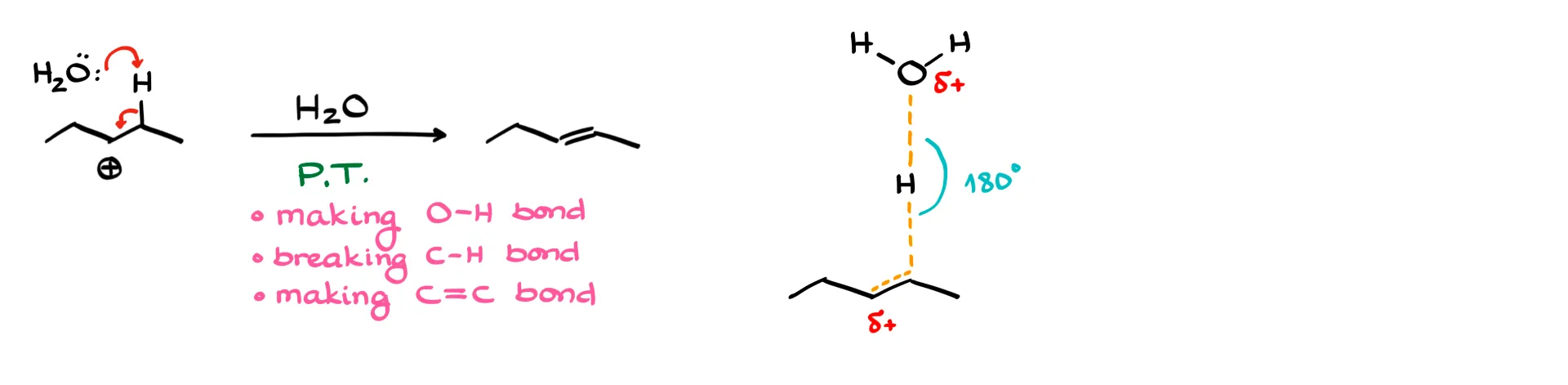
The last step forms the double bond. This can be thought of as a proton transfer, where a proton is moved from one species to another. In this step, a new oxygen–hydrogen bond is made, a carbon–hydrogen bond is broken, and a carbon–carbon π bond is formed. Again, I keep bond angles correct, even if the drawing looks awkward. Many instructors are particular about these details, so it’s worth paying attention.
A Word of Wisdom
If there’s one thing to remember, it’s this: when drawing a transition state, every curved arrow in the mechanism becomes a dashed line in the structure, representing a bond being made or broken. As long as you understand the mechanism for each step, you can draw the correct transition state.
Practice Questions
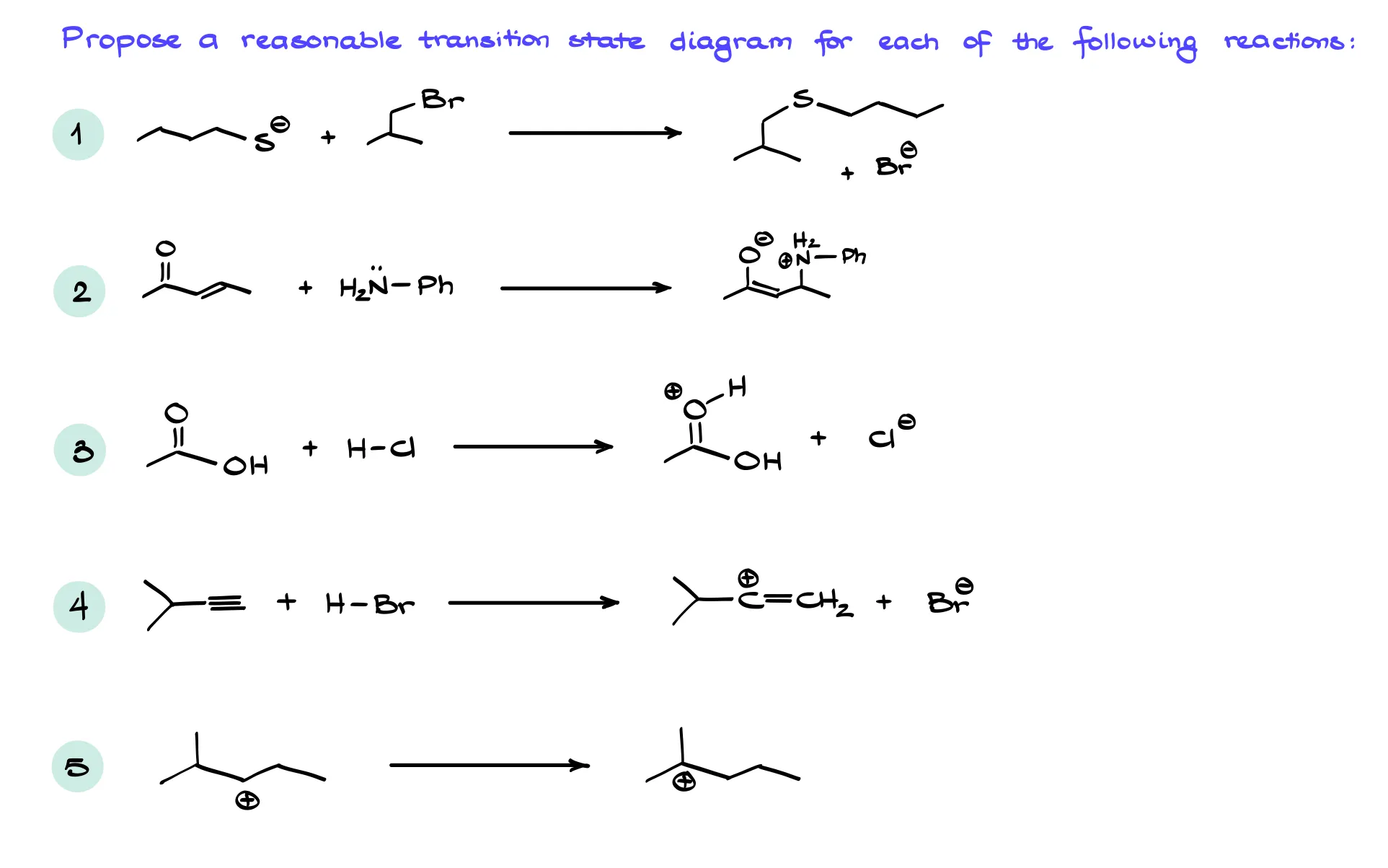
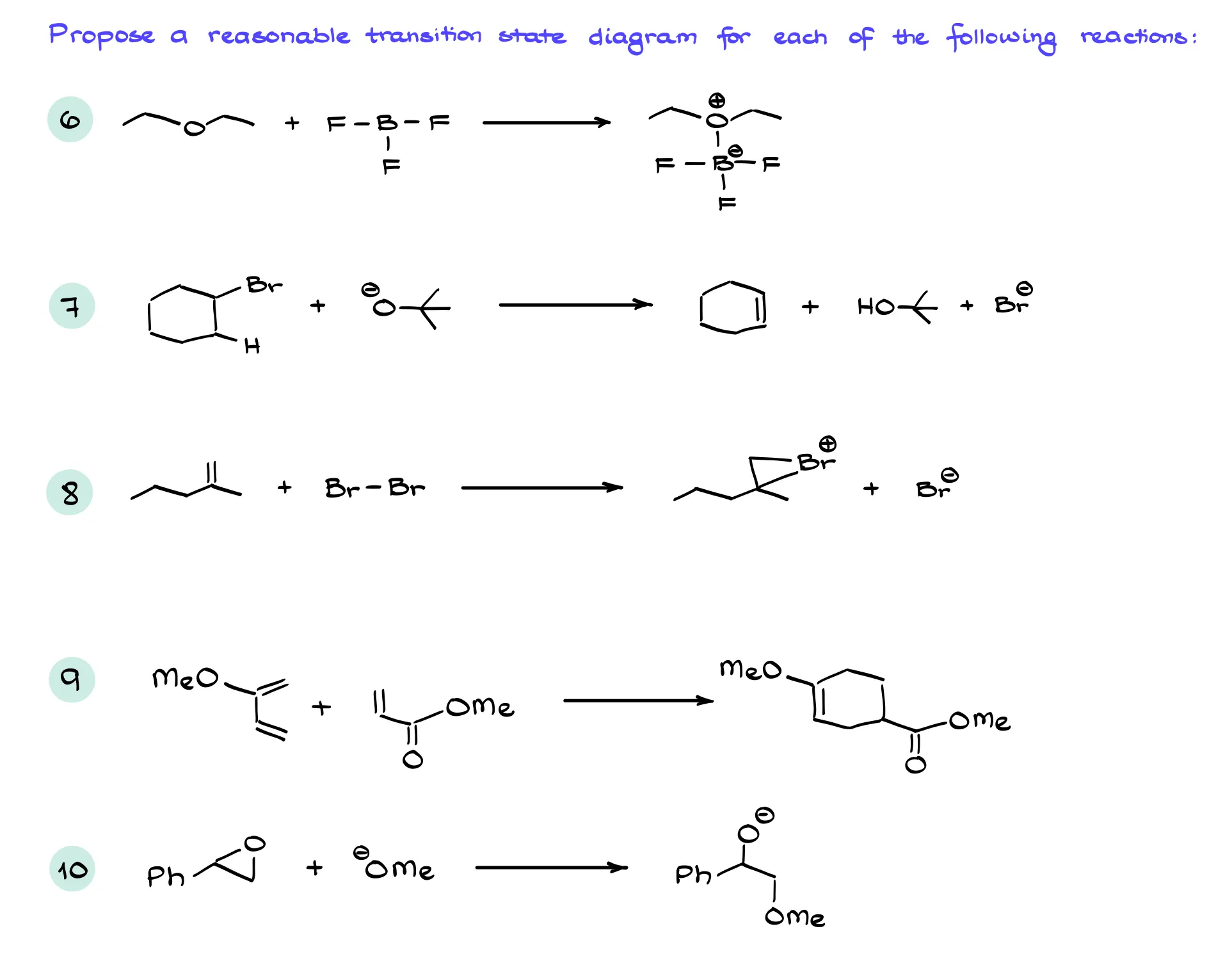
Hey there! Looks like you came across a Members-Only section.
Quizzes, Practice Workbooks, and Answer Keys, plus some tutorials are only accessible to Organic Chemistry Tutor members.
